The Effect of Oxygen on the Optical Properties of Citric Acid-Based Carbon Dots †
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
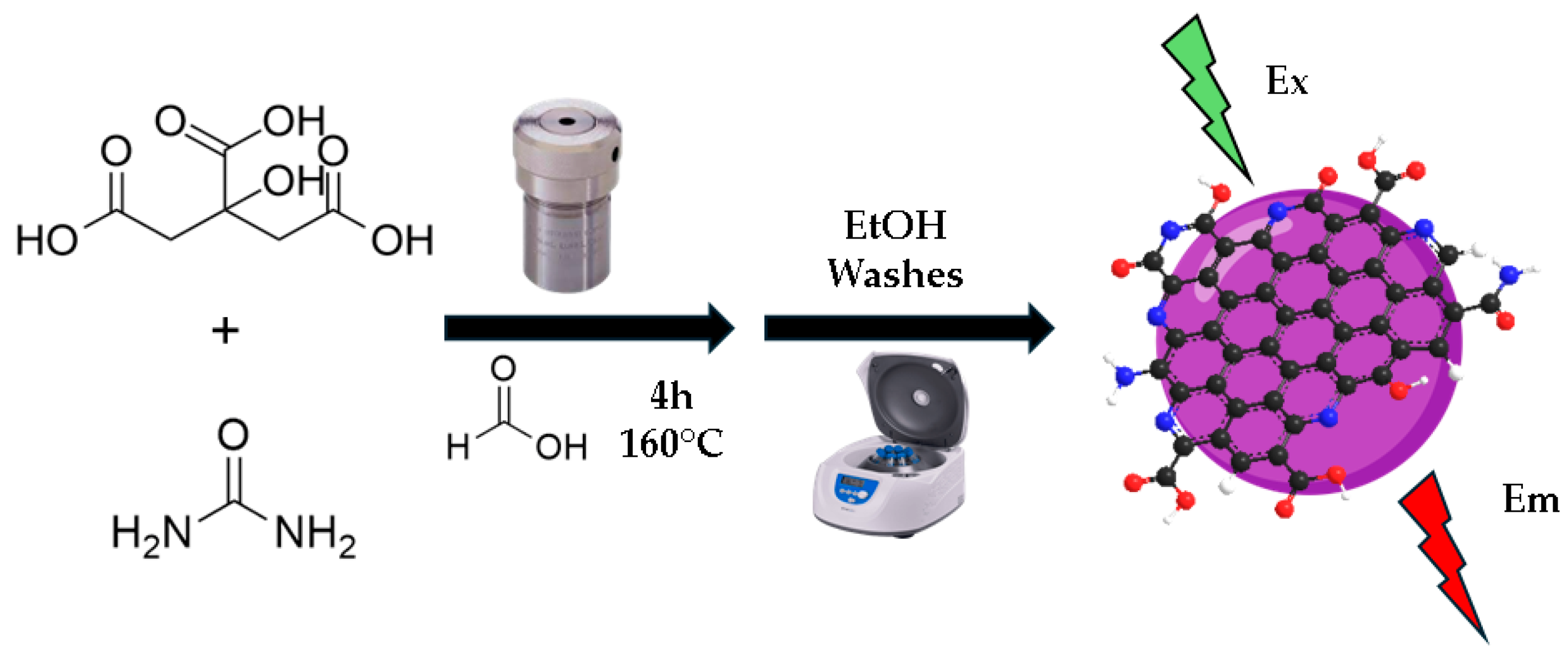
2.3. Methods
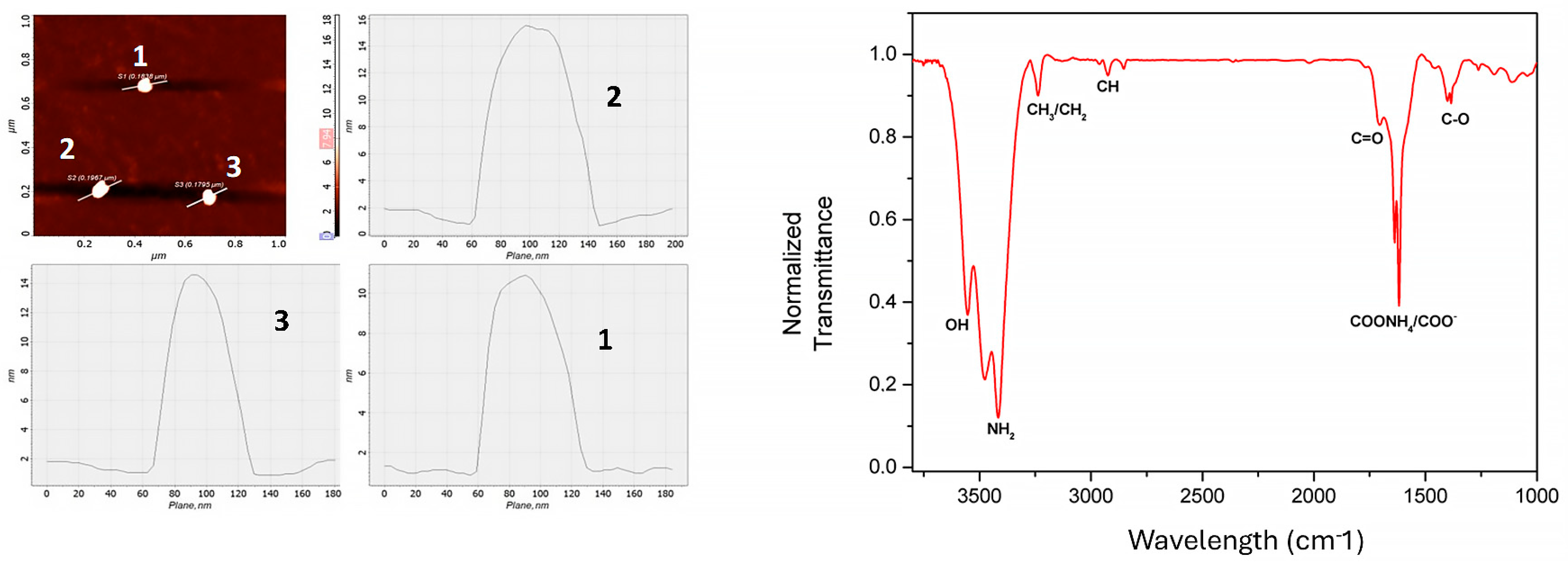
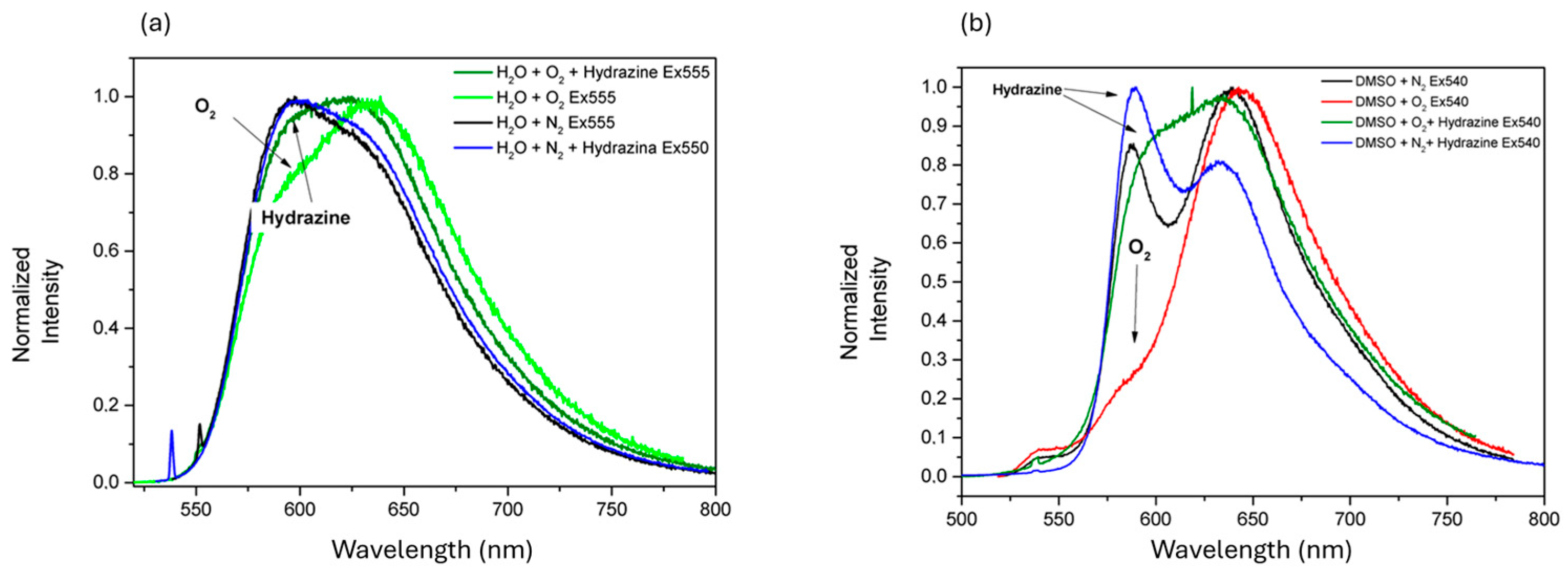
3. Results and Discussion
Characterization
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- Kang, C.; Huang, Y.; Yang, H.; Yan, X.F.; Chen, Z.P. A review of carbon dots produced from biomass wastes. Nanomaterials 2020, 10, 2316. [Google Scholar] [CrossRef]
- Liao, J.; Yao, Y.; Lee, C.H.; Wu, Y.; Li, P. In vivo biodistribution, clearance, and biocompatibility of multiple carbon dots containing nanoparticles for biomedical application. Pharmaceutics 2021, 13, 1872. [Google Scholar] [CrossRef]
- Rao, L.; Zhang, Q.; Sun, B.; Wen, M.; Zhang, J.; Zhong, G.; Fu, T.; Niu, X. Multicolor Luminescent Carbon Dots: Tunable Photoluminescence, Excellent Stability, and Their Application in Light-Emitting Diodes. Nanomaterials 2022, 12, 3132. [Google Scholar] [CrossRef]
- Hamed, M.; Chinnam, S.; Bedair, A.; Emara, S.; Mansour, F.R. Carbon quantum dots from natural sources as sustainable probes for metal ion sensing: Preparation, characterizations and applications. Talanta Open 2024, 10, 100348. [Google Scholar] [CrossRef]
- Wang, B.; Cai, H.; Waterhouse GI, N.; Qu, X.; Yang, B.; Lu, S. Carbon Dots in Bioimaging, Biosensing and Therapeutics: A Comprehensive Review. Small Sci. 2022, 2, 2200012. [Google Scholar] [CrossRef]
- Michenzi, C.; Scaramuzzo, F.; Salvitti, C.; Pepi, F.; Troiani, A.; Chiarotto, I. Photo-Activated Carbon Dots as Catalysts in Knoevenagel Condensation: An Advance in the Synthetic Field. Photochem 2024, 4, 361–376. [Google Scholar] [CrossRef]
- Carioscia, A.; Cocco, E.; Casacchia, M.E.; Gentile, G.; Mamone, M.; Giorgianni, G.; Incerto, E.; Prato, M.; Pesciaioli, F.; Filippini, G.; et al. Nitrogen-Rich Carbon Dots as Effective Catalysts in the 1,4-Reduction of α,β-Unsaturated Aldehydes via Ion Pair Asymmetric Nano-Organocatalysis. ACS Catal. 2024, 14, 13429–13438. [Google Scholar] [CrossRef]
- Nguyen, V.-N.; Pham, H.L.; Nguyen, X.T. Recent progress in organic carbon dot-based photosensitizers for photodynamic cancer therapy. Dye. Pigment. 2024, 230, 112359. [Google Scholar] [CrossRef]
- Karagianni, A.; Tsierkezos, N.G.; Prato, M.; Terrones, M.; Kordatos, K.V. Application of carbon-based quantum dots in photodynamic therapy. Carbon 2023, 203, 273–310. [Google Scholar] [CrossRef]
- Ardini, B.; Manzoni, C.; Squeo, B.; Villafiorita-Monteleone, F.; Grassi, P.; Pasini, M.; Bollani, M.; Virgili, T. Spectral Imaging of UV-Blocking Carbon Dot-Based Coatings for Food Packaging Applications. Coatings 2023, 13, 785. [Google Scholar] [CrossRef]
- Lagonegro, P.; Giovanella, U.; Pasini, M. Carbon Dots as a Sustainable New Platform for Organic Light Emitting Diode. Coatings 2020, 11, 5. [Google Scholar] [CrossRef]
- Chen, M.; Ma, J.; Chen, C.; Ding, J.; Liu, Y.; He, H.; Liu, Q.; Hu, G.; Wu, Y.; Liu, X. Cutting-edge innovations in red carbon dots: Synthesis, perfection, and breakthroughs in optoelectronics and electrocatalysis. Chem. Eng. J. 2024, 498, 155302. [Google Scholar] [CrossRef]
- Qin, W.; Wang, M.; Li, Y.; Li, L.; Abbas, K.; Li, Z.; Tedesco, A.C.; Bi, H. Recent advances in red-emissive carbon dots and their biomedical applications. Mater. Chem. Front. 2024, 8, 930–955. [Google Scholar] [CrossRef]
- Warjurkar, K.; Panda, S.; Sharma, V. Red emissive carbon dots: A promising next-generation material with intracellular applicability. J. Mater. Chem. B 2023, 11, 8848–8865. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.M.; Khan, W.U.; Ahmed, F.; Wei, Y.; Xiong, H. Recent developments of Red/NIR carbon dots in biosensing, bioimaging, and tumor theranostics. Chem. Eng. J. 2023, 465, 143010. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Zhao, S.; Wang, B.; Song, X.; Xiao, J.; Lan, M. Synthesis strategies, luminescence mechanisms, and biomedical applications of near-infrared fluorescent carbon dots. Coord. Chem. Rev. 2022, 470, 214703. [Google Scholar] [CrossRef]
- Jiang, L.; Ding, H.; Xu, M.; Hu, X.; Li, S.; Zhang, M.; Zhang, Q.; Wang, Q.; Lu, S.; Tian, Y.; et al. UV–Vis–NIR Full-Range Responsive Carbon Dots with Large Multiphoton Absorption Cross Sections and Deep-Red Fluorescence at Nucleoli and In Vivo. Small 2020, 16, 2000680. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, G.; Zhang, Z.; Lei, J.H.; Liu, T.-M.; Xing, G.; Deng, C.-X.; Tang, Z.; Qu, S. One step synthesis of efficient red emissive carbon dots and their bovine serum albumin composites with enhanced multi-photon fluorescence for in vivo bioimaging. Light Sci. Appl. 2022, 11, 113. [Google Scholar] [CrossRef]
- Liu, E.; Liang, T.; Ushakova, E.V.; Wang, B.; Zhang, B.; Zhou, H.; Xing, G.; Wang, C.; Tang, Z.; Qu, S.; et al. Enhanced Near-Infrared Emission from Carbon Dots by Surface Deprotonation. J. Phys. Chem. Lett. 2021, 12, 604–611. [Google Scholar] [CrossRef]
- Shi, W.; Yao, Q.; Qu, S.; Chen, H.; Zhang, T.; Chen, L. Micron-thick highly conductive PEDOT films synthesized via self-inhibited polymerization: Roles of anions. NPG Asia Mater. 2017, 9, e405. [Google Scholar] [CrossRef]
- Yemata, T.A.; Zheng, Y.; Kyaw AK, K.; Wang, X.; Song, J.; Chin, W.S.; Xu, J. Modulation of the doping level of PEDOT:PSS film by treatment with hydrazine to improve the Seebeck coefficient. RSC Adv. 2020, 10, 1786–1792. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Zhu, S.; Feng, T.; Xia, C.; Song, Y.; Yang, B. The polymeric characteristics and photoluminescence mechanism in polymer carbon dots: A review. Mater. Today Chem. 2017, 6, 13–25. [Google Scholar] [CrossRef]
- Xia, C.; Zhu, S.; Feng, T.; Yang, M.; Yang, B. Evolution and Synthesis of Carbon Dots: From Carbon Dots to Carbonized Polymer Dots. Adv. Sci. 2019, 6, 1901316. [Google Scholar] [CrossRef]
- Mondal, M.; Pramanik, S. A mechanism for excitation-dependent emission from carbon nanodots. Mater. Lett. X 2023, 18, 100195. [Google Scholar] [CrossRef]
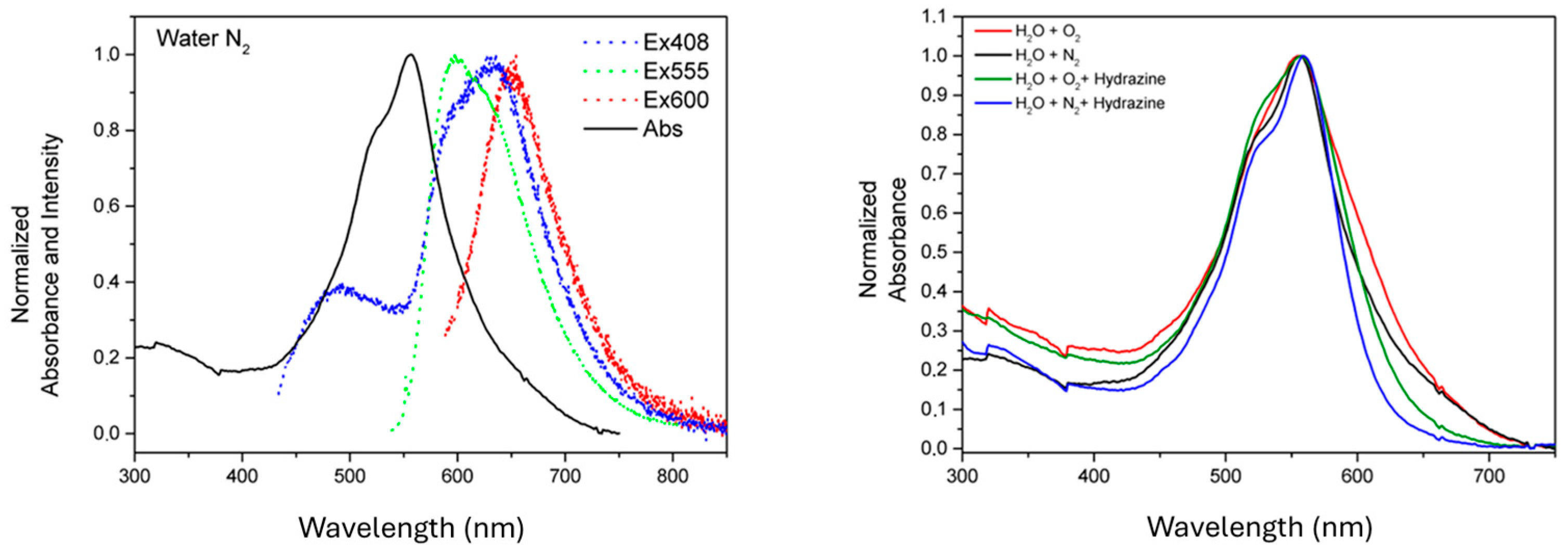
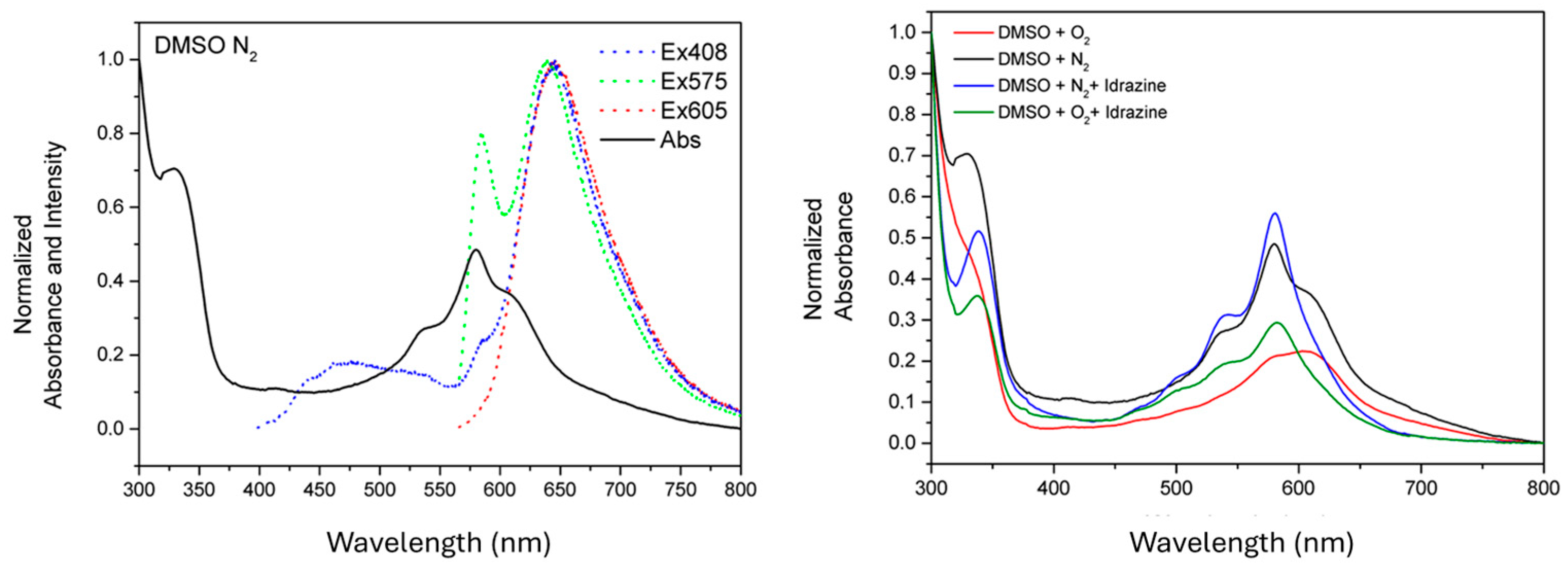
| N2 QY% | O2 QY% | O2 Hydrazine QY% | N2 Hydrazine QY% | |
|---|---|---|---|---|
| H2O | DMSO | DMSO | DMSO | DMSO |
| 15 | 54 | 45 | 30 | 47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turco, F.; Squeo, B.M.; Villafiorita-Monteleone, F.; Botta, C.; Pasini, M. The Effect of Oxygen on the Optical Properties of Citric Acid-Based Carbon Dots. Chem. Proc. 2024, 16, 107. https://doi.org/10.3390/ecsoc-28-20195
Turco F, Squeo BM, Villafiorita-Monteleone F, Botta C, Pasini M. The Effect of Oxygen on the Optical Properties of Citric Acid-Based Carbon Dots. Chemistry Proceedings. 2024; 16(1):107. https://doi.org/10.3390/ecsoc-28-20195
Chicago/Turabian StyleTurco, Federico, Benedetta Maria Squeo, Francesca Villafiorita-Monteleone, Chiara Botta, and Mariacecilia Pasini. 2024. "The Effect of Oxygen on the Optical Properties of Citric Acid-Based Carbon Dots" Chemistry Proceedings 16, no. 1: 107. https://doi.org/10.3390/ecsoc-28-20195
APA StyleTurco, F., Squeo, B. M., Villafiorita-Monteleone, F., Botta, C., & Pasini, M. (2024). The Effect of Oxygen on the Optical Properties of Citric Acid-Based Carbon Dots. Chemistry Proceedings, 16(1), 107. https://doi.org/10.3390/ecsoc-28-20195








