Abstract
The beneficial health effects of pomegranate have consistently garnered scientific interest. This study delves into the health-promoting qualities of pomegranate seed oil from northwest Algeria, renowned for its nutraceutical benefits. Through Soxhlet extractions, oil yields between 11% and 17% were achieved, dependent on the chosen solvent. The oil's quality was rigorously assessed using indicators like iodine, acid, and saponification indices. Gas chromatography identified the predominance of unsaturated fatty acids (comprising 95% of the total), with a notable presence of punicic acid at 83.20%. This exceptionally high punicic acid content establishes Beni-Snouss pomegranate seed oil as a global punicic acid treasure trove, underscoring its significance as a valuable nutraceutical resource.
1. Introduction
Pomegranate trees (Punica granatum L.) are originally from Asia. They spread gradually to various regions [1], including the Mediterranean basin. In Algeria, pomegranates flourish throughout the country. The one that caught our attention is that of Beni-Snouss, a mountainous region of 835 m altitude at 34°38′35″ N and 1°33′41″ W, characterized by a Mediterranean climate. In this area, the Atmi variety stands out for its low consumption due to its very acidic juice and its large seeds.
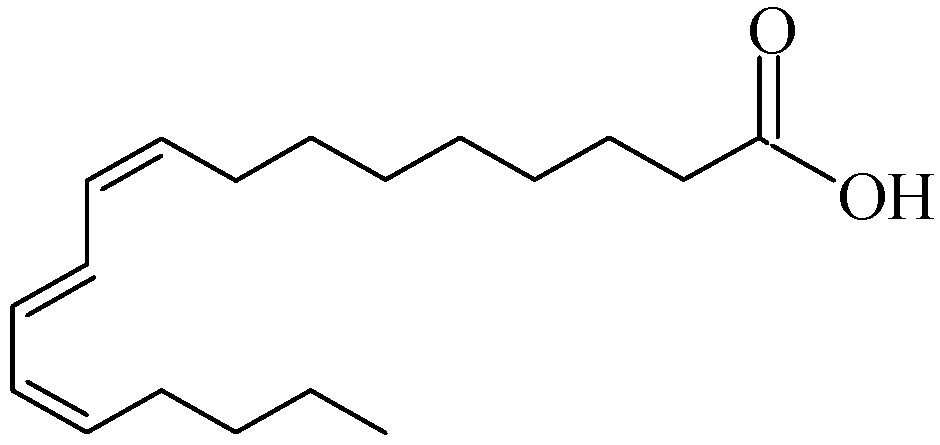
The pomegranate fruit consists of a hard pericarp and an inner membranous wall, which collectively make up 30–50% of its weight, and arils, accounting for 52% of the total fruit weight [2]. Arils contain both juice and seeds, with seed content varying from 3.7% to 14.3% of the total weight, depending on factors such as species, location, growth conditions, and maturity. Notably, approximately 80% of pomegranate seed oil (PSO) is composed of punicic acid (C18:3-9cis, 11trans, 13cis), an octadecatrienoic fatty acid that is a positional and geometric isomer of α-linolenic acid (see Figure 1).
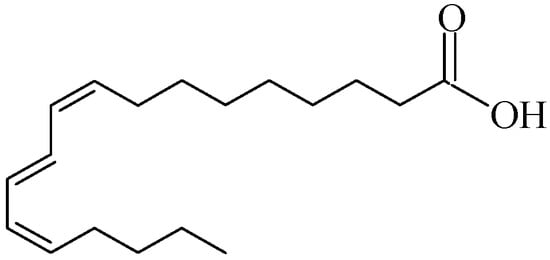
Figure 1.
Structure of punicic acid.
Punicic acid is reportedly synthesized in situ from linoleic acid [3]. Not surprisingly, linoleic acid is also present (7%), along with stearic acid (2.6%), primarily in the form of triglycerides. Other constituents include sterols, steroids, and cerebroside, accounting for 2–8% of the total seed weight.
While pomegranate seeds have traditionally been considered waste in the pomegranate juice industry, recent reports have highlighted their medicinal significance [4,5,6]. These seeds exhibit various benefits, such as antidiabetic and anticancer activities, particularly against hormonal cancers, as described by several groups [5]. This prompted our unique study of Algerian pomegranate seed oil (PSO) for the first time, employing different extraction techniques and multiple analyses.
2. Materials and Methods
2.1. Vegetable Matter
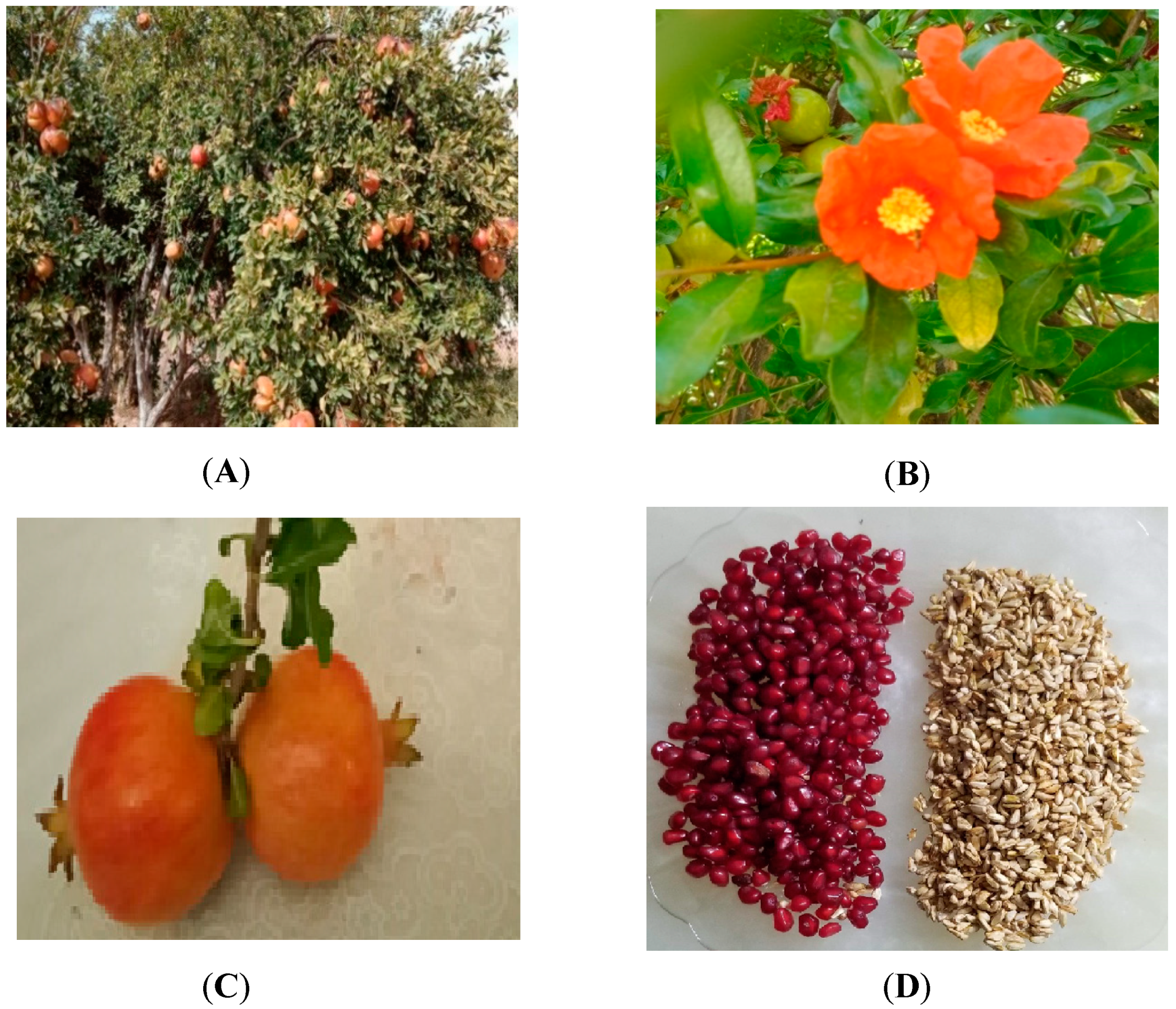
As previously reported, Punica granatum L. fruits are harvested from the Beni-Snouss region (Figure 2A). Their blossoming occurs at the end of May (Figure 2B). The fruits (Figure 2C) undergo water washing and manual peeling. The resulting arils (Figure 2D) are pressed to extract the juice, and the seeds are washed with distilled water before being dried at temperatures ranging from 40 to 60 °C until their weight stabilizes. Subsequently, they are manually ground in a mortar and sifted using a 60-mesh sieve to achieve a uniform particle distribution with a size of 0.25 mm.
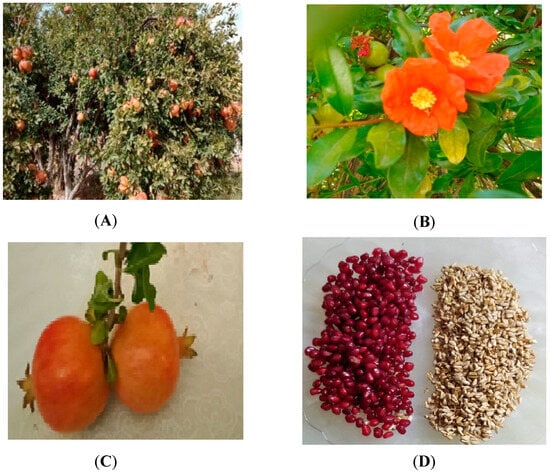
Figure 2.
Punica granatum: Atmi. (A): plant habit; (B): flowering stem; (C): fruit; (D): seeds with aril.
2.2. Reagents and Chemicals
All the solvents and chemicals employed were of analytical grade, obtained from Sigma-Aldrich (Schnelldorf, Germany), and used as procured.
2.3. Oil Extraction: Soxhlet
Two protocols were employed for this extraction:
- Method A: A total of 20 g of Punica granatum seeds are placed in a Soxhlet apparatus with a flask containing 200 mL of solvent. Extraction is performed over 8 h. Afterward, the mixture is transferred to the rotary evaporator for complete solvent evaporation. The isolated oil is stored at −4 °C, and the yield is expressed as a percentage of the isolated oil weight relative to the weight of the ground seeds.
- Method B: In this modified protocol designed to shorten the extraction time, 20 g of Punica Granatum seeds are loaded into the extraction cartridge, which is then inserted into a flask containing 200 mL of solvent. After 30 min of refluxing, both the solvent and cartridge are transferred into a Soxhlet apparatus and refluxed for an additional 60 min. The mixture is then transferred to the rotary evaporator for complete solvent evaporation. The isolated oil is stored at −4 °C, and the yield is expressed as a percentage of the isolated oil weight relative to the weight of the ground seeds.
2.4. Physico-Chemical Analysis: Oil Quality Parameters
Official methods, specifically AOCS methods [7], were adhered to for assessing the oil's quality. These included AOCS Cd 3d-63 protocols for acid value (AV), AOCS Method Cd 1b-87 (97) for iodine value (IV), and peroxide index measurement in accordance with AOCS Method Cd 8b-90 (97) (AOCS, 1997). ISO 3657:1988 was employed for determining the saponification index. Additionally, the PSO refractive index was determined at 25 °C using an Abbe Refractometer. Density measurements were conducted at 25 °C using a 10 mL pycnometer, while viscosity was assessed at 25 °C with a SCHOTT GERATE AVS 400 capillary viscometer.
2.5. High-Performance Thin-Layer Chromatography (HPTLC)—Lipid Classes
The lipid classes of PSO were analyzed using high-performance thin-layer chromatography (HPTLC) combined with scanning densitometry, following a previously described method [8]. Chromatography involved two distinct developments aimed at separating the polar and neutral classes.
2.6. Analysis by Gas Chromatography (GC)-FID
2.6.1. Preparation of Fatty Acid Methyl Esters (FAMEs)
Fatty acid methyl esters (FAME) were prepared and subsequently analyzed using gas chromatography (GC) [9].
2.6.2. FAMEs GC-FID Analysis
The fatty acid methyl esters (FAME) were prepared as described in the literature and identified using GC using a clay apparatus (Kyoto, Japan) equipped with a capillary column BD-EN14103 (Agilent) sized 30 m × 320 µm × 0.25 µm. Helium was used as the carrier gas at 33 cm·s−1 [8].
3. Results and Discussion
3.1. Localization and Quantification
The bark of the Atmi variety accounts for 36.2% of the total fruit weight, while arils make up 62.5% of the total fruit. Through mechanical pressing, one can obtain up to 56.3% of the total fruit weight as acidic juice. The remaining seeds constitute 6.1% of the total fruit weight after washing and drying. Overall, Atmi is highly comparable to the Spanish varieties Valenciana and Mollar Elche [10], as shown in Table 1.

Table 1.
Comparison of the variety Atmi with Spanish varieties.
3.2. Soxhlet Approach to Oil-Extraction
The extraction of oil from Atmi’s seeds was carried out using a Soxhlet apparatus with various solvents, following the previously described methods A and B. The results have been compiled in Table 2.

Table 2.
Total lipid yields of Atmi’s oil extraction from seeds with methods A and B.
The yields obtained through both methods are nearly identical, although the latter method is more advantageous due to its time efficiency (90 min vs. 8 h for the first method).
3.3. PSO Physico-Chemical Indices
The physico-chemical analysis of oils is crucial as it reflects their nutraceutical and pharmaceutical value. This is often achieved by measuring important indices, as listed in Table 3.

Table 3.
Physico-chemical characteristics of Atmi PSO.
Specifically, an acid index below 4 mg/g indicates safe oil suitable for consumption. In the case of Atmi PSO, its acid index of 2.24 confirms its high acceptability for consumers.
The peroxide index measurement for Atmi PSO yielded an average value of 1.69. This suggests excellent stability against oxidation and longer preservation.
The refractive index of 1.517 determined for Atmi PSO is relevant and complies with CODEX standards for oils with a high degree of unsaturation [11].
3.4. Chemical Composition of the PSO
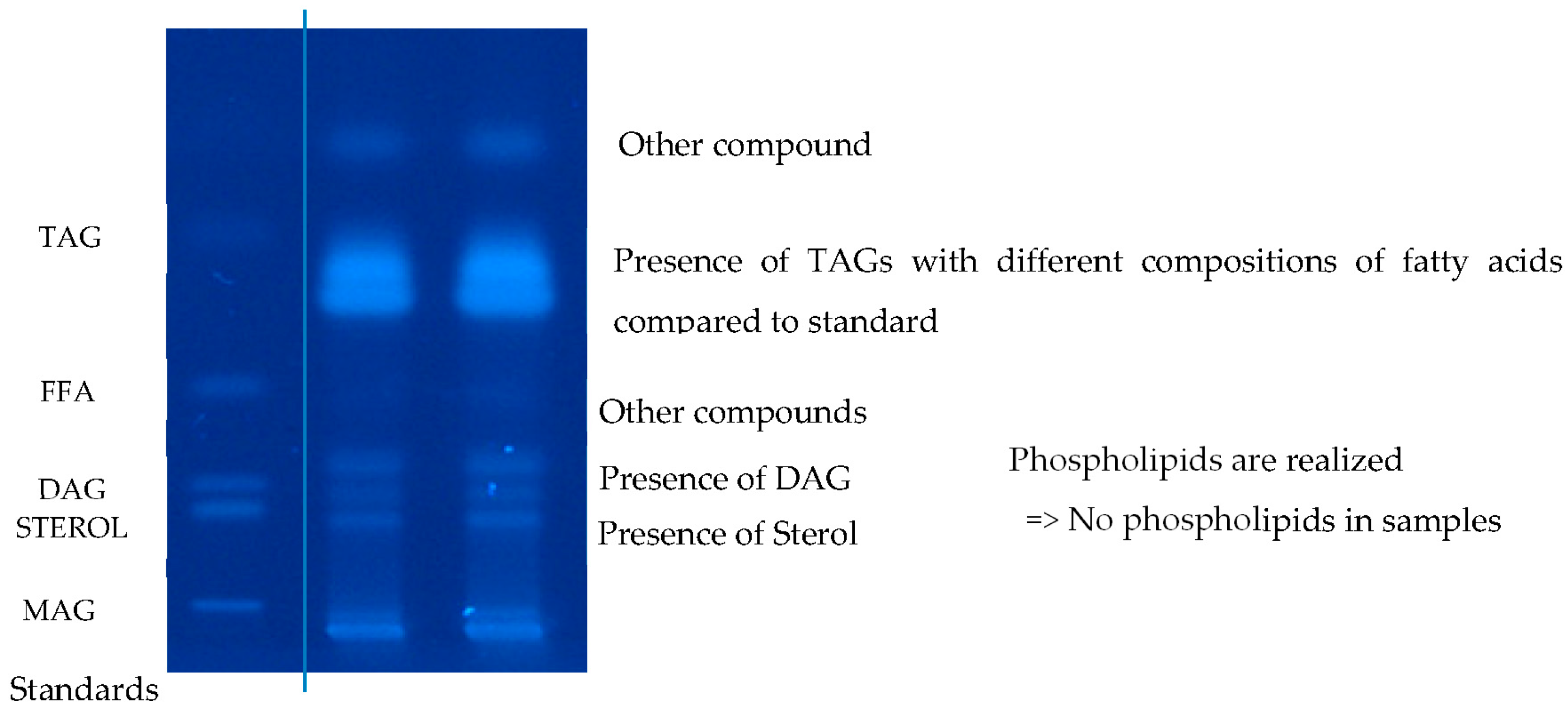
The PSO extracts underwent analysis using high-performance thin-layer chromatography (HPTLC) to identify various lipid classes. The examination of the extracted oils revealed that triglycerides (TAGs) constituted over 99% of the total lipids, while diacylglycerides (DAGs) represented only trace amounts, approximately 0.59–1% (Figure 3). These findings align perfectly with those previously reported by the Kaufman team [12].
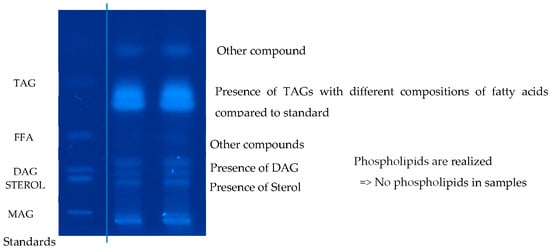
Figure 3.
Relative profile of lipid classes based on high-performance thin-layer chromatography.
All extracted lipids were converted into FAME derivatives, and then fatty acids were separated and identified using gas chromatography coupled with a flame ionization detector (GC-FID). The data obtained indicated a high content of polyunsaturated fatty acids (PUFA), accounting for 90% of the total weight, in contrast to just 6% for monounsaturated fatty acids (MUFA) (Table 4).

Table 4.
Chemical composition and relative amounts of PSO fatty acids.
The ratio of saturated fatty acids to the total unsaturated ones (SFA/(PUFA + MUFA)) is notably low at 0.043 when compared to the Spanish Mollar de Elche variety, which reportedly has a ratio of 0.079 [10]. This emphasizes the increasing significance of Atmi PSO in nutraceutical and pharmaceutical applications, attributed to its high content of total unsaturated fatty acids.
It’s worth noting that PSO has demonstrated substantial inhibitory effects on tumoral cell growth [4,13]. Additionally, pomegranate seed oil has been found to prevent obesity induced by a high-fat diet and to improve insulin sensitivity, reducing the risk of type 2 diabetes [6].
GC analysis of PSO highlighted two major compounds, namely punicic acid (C18:3) and linoleic acid (C18:2) (Table 5). Together, they constitute more than 89% of the total fatty acid composition of the extracted oils (Table 5), with oleic acid being the third most abundant fatty acid at 5.69%.

Table 5.
Yields (%) of fatty acid composition and lipid classes extracted from Atmi pomegranate seeds using the Soxhlet method and hexane as solvent.
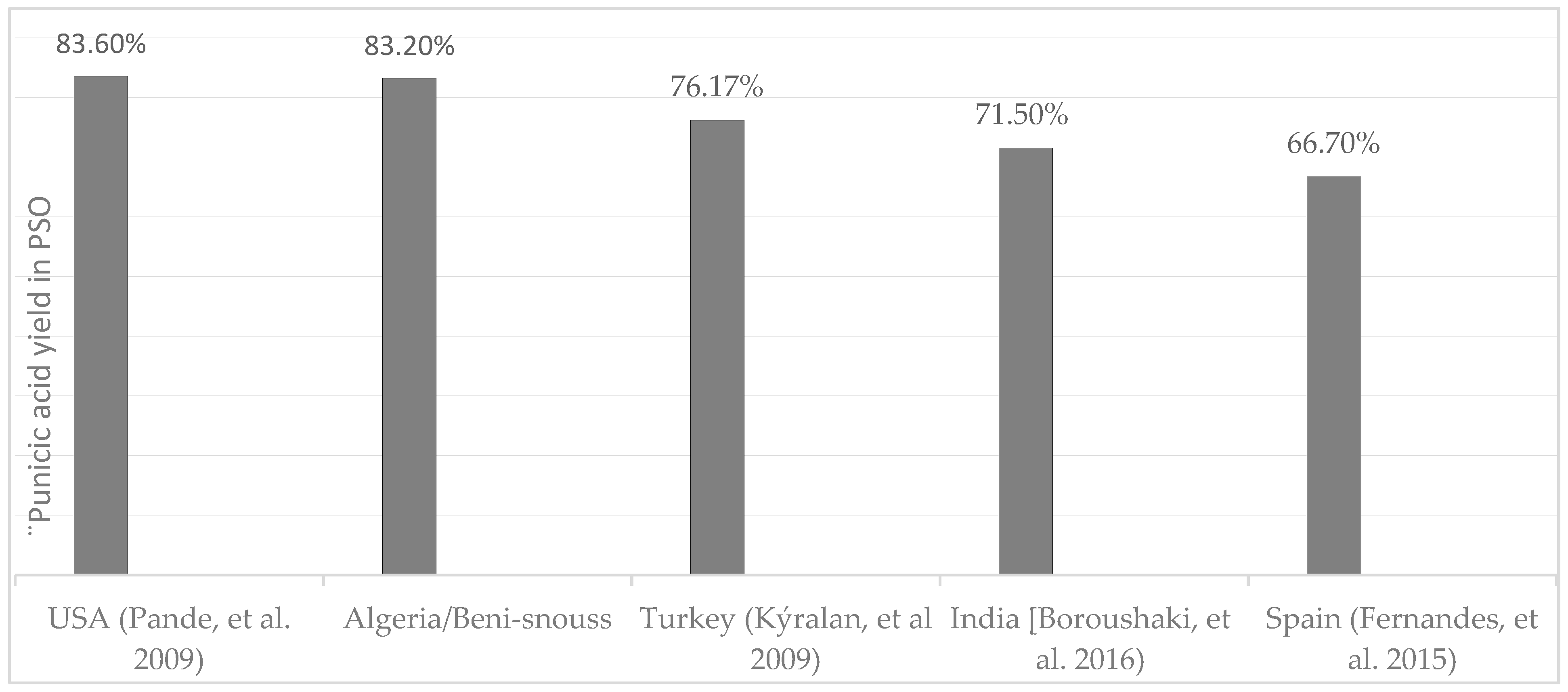
Our findings have highlighted punicic acid (PA) as the predominant constituent of the fatty acids, comprising a remarkable 83.20% of the composition. In comparison, PSO of Turkish origin contains approximately 76% punicic acid [14], while that from Georgia, USA, can reach up to 83% [15], a level akin to the Atmi variety (Figure 4). Consequently, the Atmi variety is exceptionally rich in mono- and polyunsaturated fatty acids, with a total content of 95.84%.
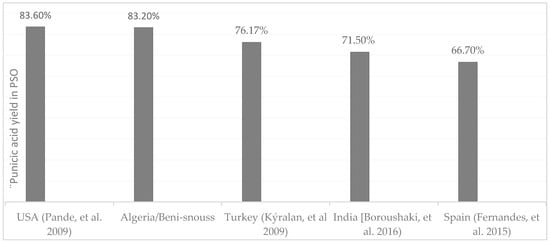
Figure 4.
Punicic acid content in different varieties by country [5,10,14,15].
Gondoic acid (11-eicosenoic acid), an omega-9 fatty acid commonly found in jojoba oil, and oleic acid (C18:1 n-9) are the only monounsaturated fatty acids present in PSO, accounting for 0.43% and 5.69% of the total weight, respectively. Additionally, palmitic acid at 2.43% and stearic acid at 1.73% are the only fully saturated fatty acids. While their presence is minor among other fatty acids, their contents are comparable to those in pomegranates originating from Turkey (2.1–2.77% and 1.35–2.01%) [14], yet lower than those harvested in Georgia, USA (2.8–4.8% and 2.1–3.6%) [15].
Punicic acid (PA) emerges as the predominant ingredient in Atmi PSO, and its nutraceutical significance cannot be overstated. It enhances the immune system through its anti-inflammatory and antioxidant properties [5].
Finally, the high levels of punicic acid and unsaturated fatty acids in Atmi PSO, as reported here, render this product extremely valuable in the pharmaceutical and cosmetic industries.
Author Contributions
All authors contributed equally to this work. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors are grateful to the General Directorate for Scientific Research and Technological Development (DGRSDT) and the University of Tlemcen.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Verma, N.; Mohanty, A.; Lal, A. Pomegranate Genetic Resources and Germplasm Conservation. A Review. Fruit Veg. Cereal Sci. Biotechnol. 2010, 4, 120–125. [Google Scholar]
- Mohagheghi, M.; Rezaei, K.; Labbafi, M.; Mousavi, S.M.E. Pomegranate seed oil as a functional ingredient in beverages. Eur. J. Lipid Sci. Technol. 2011, 113, 730–736. [Google Scholar] [CrossRef]
- de Melo, I.L.P.; Carvalho, E.B.T.; Mancini-Filho, J. Pomegranate Seed Oil (Punica granatum L.): A Source of Punicic Acid (Conjugated α-Linolenic Acid). J. Hum. Nutr. Food Sci. 2014, 2, 1024–1035. [Google Scholar]
- Holic, R.; Xu, Y.; Caldo, K.M.P.; Singer, S.D.; Field, C.J.; Weselake, R.J.; Chen, G. Bioactivity and biotechnological production of punicic acid. Appl. Microbiol. Biotechnol. 2018, 102, 102–3537. [Google Scholar] [CrossRef] [PubMed]
- Boroushaki, M.T.; Mollazadeh, H.; Afshari, A.R. Pomegranate seed oil: A comprehensive review on its therapeutic effects. Int. J. Pharm. Sci. Res. 2016, 7, 430–442. [Google Scholar]
- Vroegrijk, I.O.; van Diepen, J.A.; van den Berg, S.; Westbroek, I.; Keizer, H.; Gambelli, L.; Havekes, L.M. Pomegranate seed oil, a rich source of punicic acid, prevents diet-induced obesity and insulin resistance in mice. Food Chem. Toxicol. 2011, 49, 1426–1430. [Google Scholar] [CrossRef] [PubMed]
- AOCS Official Methods of Analysis. Methods Cd 3d-63; Cd 1b-87; Cd 8b-90; AOCS: Champaign, IL, USA, 1997. [Google Scholar]
- Ben-Youssef, S.; Fakhfakh, J.; Breil, C.; Abert-Vian, M.; Chemat, F.; Allouche, N. Green extraction procedures of lipids from Tunisian date palm seeds. Ind. Crops Prod. 2017, 108, 520–525. [Google Scholar] [CrossRef]
- Morrison, W.R.; Smith, L.M. Preparation of fatty acid methyl esters and dimethyl acetals from lipids with boron fluoridemethanol. J. Lipid Res. 1964, 5, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.; Pereira, J.A.; Lopéz-Cortés, I.; Salazar, D.M.; Ramalhosa, E.; Casal, S. Fatty acid, vitamin E and sterols composition of seed oils from nine different pomegranate (Punica granatum L.) cultivars grown in Spain. J. Food Compos. Anal. 2015, 39, 13–22. [Google Scholar] [CrossRef]
- Codex Alimentarius. 2001. Codex standard for named vegetable oils cx-stan 210. Named Veg. Oils 1999, 8, 11–25. [Google Scholar]
- Kaufman, M.; Wiesman, Z. Pomegranate Oil Analysis with Emphasis on MALDI-TOF/MS Triacylglycerol Fingerprinting. J. Agric. Food Chem. 2007, 55, 10405–10413. [Google Scholar] [CrossRef] [PubMed]
- Aruna, P.; Venkataramanamma, D.; Singh, A.K.; Singh, R.P. Health Benefits of Punicic Acid. A Review. Compr. Rev. Food Sci. Food Saf. 2014, 15, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Kýralan, M.; Gölükcü, M.; Tokgöz, H. Oil and Conjugated Linolenic Acid Contents of Seeds from Important Pomegranate Cultivars (Punica granatum L.) Grown in Turkey. J. Am. Oil Chem. Soc. 2009, 86, 985–990. [Google Scholar] [CrossRef]
- Pande, G.; Akoh, C.C. Antioxidant Capacity and Lipid Characterization of Six Georgia-Grown Pomegranate Cultivars. J. Agric. Food Chem. 2009, 57, 9427–9436. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).