Abstract
A semi-synthetic strategy and a new operationally simple-to-obtain heterocyclic system, imidazo-5α-hydroxyvouacapane, was developed in two-step reactions. The first reaction step is a Vilsmeier–Haack formylation at the furan ring of the 5α-hydroxyvouacapane isolated from Caesalpinia pulcherrima stems to obtain the 5α-hydroxyvouacapane-aldehyde in 33% yield. The second reaction step is a Groebke–Blackburn–Bienaymé reaction to synthesize the imidazo-5α-hydroxyvouacapane by using 2-aminopyridine and tert-butyl isocyanide in 75% yield. This work contributes significantly to research on semi-synthesis through multicomponent reactions, an area with limited coverage in the literature.
1. Introduction
Distinguished by their structural diversity and complexity [1], natural products or secondary metabolites have assumed a pivotal role in pharmaceutical research [2], primarily through their isolation, facilitating the discovery of bioactive compounds indispensable in drug development [3]. Nevertheless, these metabolites rarely find direct application without modification, necessitating the development of studies on semi-synthesis [4], a scarcely explored research area that has been instrumental in understanding the synthesis of numerous derivatives with superior biological activities compared to their natural counterparts [5]. Thus, using powerful, rapid, and efficient synthetic tools becomes imperative to access these valuable natural product derivatives. Among them, isocyanide–multicomponent reactions (I-MCR) have gained prominence recently, particularly in synthesizing triterpenes and marine natural product derivatives [6,7]. However, the sparse research on these reactions makes this an enticing topic for further investigation.
On the other hand, the genus Caesalpinia has a variety of secondary metabolites, such as diterpenes, triterpenes, flavonoids, aromatic phenols, phenylpropanoids, and others. Several Caesalpinia species have found utility in traditional medicine, with compounds isolated from these plants displaying significant biological activities [8]. Among these species, Caesalpinia pulcherrima, a small tropical tree commonly used as an ornamental plant [9], has yielded metabolites with documented cytotoxic, antituberculosis, antibacterial, and antifungal properties. Isovouacapenol C 1 [10], pulcherrimin C 2 [11], pulcherrimin A 3 [12], 8,9,11,14-didehydrovouacapen-5α-ol 4, and 5α-hydroxyvouacapane 5 [10] are some of the metabolites isolated in this species, the latter being the natural product under study in this work (Figure 1).
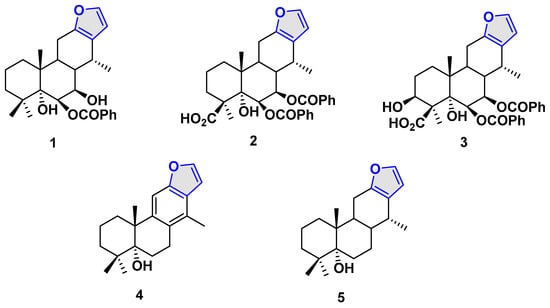
Figure 1.
Vouacapanes isolated from Caesalpinia pulcherrima.
In continuation of our studies of natural product derivatives and the synthesis of biologically relevant compounds by using I-MCR such as Ugi-azide and the Groebke–Blackburn–Bienaymé reaction (GBB) [13,14,15,16], herein, we report the isolation of 5α-hydroxyvouacapane 5 and the semi-synthesis of imidazo-5α-hydroxyvouacapane through the GBB reaction.
2. Materials and Methods
2.1. Experimental Section
All reagents, reactants, and solvents were purchased from Merck (Darmstadt, Germany), without further purification. Thin-layer chromatography (TLC) was performed with silica gel plates from Merck (silica gel 60 F 254), and a mixture of hexanes–EtOAc was used as eluent. The NMR spectra were recorded at 400 MHz for 1H and 100 MHz for 13C on a Varian Mercury 400 spectrometer (Varian, Palo Alto, CA, USA), using CDCl3 as the solvent and TMS as the internal standard. The chemical shift (δ) is reported in ppm, and the J values are given in Hertz. HRMS spectra were acquired on a Bruker MicroTOF-II spectrometer (Bruker Daltonics, Bremen, Germany). The chemical names and drawings were obtained using ChemDraw Professional (version 15.0.0.106).
2.2. Plant Material
Specimens of Caesalpinia pulcherrima were collected from Huetamo de Núñez, Michoacán state, Mexico, in October 2022.
2.3. Extraction and Isolation
The air-dried stems (500 g) of C. pulcherrima were treated under reflux with CH2Cl2 (5 L) for 12 h. The filtration and evaporation of the CH2Cl2 extract afforded a brown viscous oil (40 g, 8%), from which a portion (10 g) was subjected to silica gel column chromatography (cc) with hexanes–CH2Cl2 mixtures as the eluent. Fractions 20–25 (hexanes–CH2Cl2; 95:5) yielded purified vouacapane 5 (96 mg).
Colorless crystals; m.p. 98–100 °C; 1H-NMR (400 MHz, CDCl3): δ 7.21 (d, J = 1.1 Hz, 1H), 6.18 (d, J = 1.6 Hz, 1H), 2.58 (dd, J = 6.8, 4.1 Hz, 1H), 2.49–2.43 (m, 1H), 2.37–2.35 (m, 1H), 2.35–2.32 (m, 1H), 1.85–1.82 (m, 1H), 1.81–1.80 (m, 1H), 1.80–1.77 (m, 1H), 1.68–1.66 (m, 1H), 1.66–1.65 (m, 1H), 1.61–1.58 (m, 1H), 1.59–1.56 (m, 1H), 1.49–1.46 (m, 1H), 1.44–1.42 (m, 1H), 1.39–1.35 (m, 1H), 1.20–1.17 (m, 1H), 1.08 (s, 3H), 1.06 (s, 3H), 1.02 (d, J = 7.0 Hz, 3H), 0.95 (s, 3H). 13C-NMR (100 MHz, CDCl3): δ 149.7, 140.2, 122.5, 109.5, 76.8, 41.1, 38.4, 37.6, 36.3, 34.5, 32.4, 31.5, 28.0, 25.6, 24.7, 24.4, 22.3, 18.2, 17.5, 17.6.
2.4. Synthesis of (4aR,7R,11bR)-9-(3-(Tert-butylamino)imidazo[1,2-a]pyridin-2-yl)-4,4,7,11b-tetramethyl-1,3,4,5,6,6a,7,11,11a,11b-decahydrophenanthro[3,2-b]furan-4a(2H)-ol
The 5α-hydroxyvouacapane 5 (100 mg, 1.0 equiv.) was dissolved in dimethylformamide (0.5 M) in a 5 mL round-bottom flask. Next, a previously prepared mixture of POCl3 (160 µL, 6.0 equiv.) was added dropwise to DMF (80 µL, 3.5 equiv.). The reaction mixture was stirred at room temperature for 2 h. Then, the reaction mixture was cooled to 0 °C, and crushed ice was added and stirred vigorously until the formation of a precipitate, which was filtered, washed with water (5 mL), and dried under vacuum to yield a yellow powder. Finally, it was purified via flash column chromatography with hexanes–EtOAc 8:2 (v/v) to yield vouacapane-aldehyde 6 as a yellow oil (37%).
Yellow oil; RF = 0.80 (Hex:EtOAc 9:1 v/v); 1H-NMR (400 MHz, CDCl3): δ 9.47 (s, 1H), 7.05 (s, 1H), 2.67 (dd, J = 7.0, 4.4 Hz, 1H), 2.60–2.54 (m, 1H), 2.50–2.40 (m, 2H), 1.88–1.85 (m, 1H), 1.83–1.82 (m, 1H), 1.81–1.78 (m, 1H), 1.67–1.66 (m, 1H), 1.65–1.63 (m, 1H), 1.63–1.57 (m, 2H), 1.45–1.40 (m, 1H), 1.37–1.35 (m, 1H), 1.33–1.30 (m, 1H), 1.22–1.19 (m, 1H), 1.09 (s, 3H), 1.05 (d, J = 9.0 Hz, 6H), 0.95 (s, 3H). 13C-NMR (100 MHz, CDCl3): δ 176.79, 158.61, 151.45, 126.85, 122.60, 41.08, 38.32, 37.01, 36.20, 34.33, 32.36, 31.24, 29.64, 27.90, 25.52, 24.64, 24.16, 22.74, 18.06, 17.48, 16.91. HRMS (ESI+): m/z: Calcd. for C21H30O3 [M + H]+: 330.2195; found: 331.2268.
2.5. Synthesis of (5R,11bS)-9-(3-(Tert-butylamino)imidazo[1,2-a]pyridin-2-yl)-4,4,7,11b-tetramethyl-1,2,3,4,4a,5,6,11b-octahydrophenanthro[3,2-b]furan-5-yl Acetate
Vouacapane-aldehyde 6 (20 mg, 0.06 mmol), 2-aminopyridine (5.7 mg, 0.06 mmol), and Sc(OTf)3 (10% mol) were dissolved in a mixture of MeOH/DCM (0.5 M, 2:3 v/v) in a 5 mL round-bottom flask and reacted for 5 min at room temperature. Then, isocyanide (5.79 μL, 0.06 mmol) was added, and the reaction mixture was stirred at room temperature until reaction consumption (monitored by TLC). Next, the reaction mixture was evaporated under reduced pressure, and the residue was purified via flash column chromatography with hexanes–EtOAc (1:1) to afford 9 as an orange solid (21.8 mg, 74%).
Solid orange; mp = 94–97 C RF = 0.20 (Hex:EtOAc 7:3 v/v); 1H-NMR (400 MHz, CDCl3): δ 8.20 (dt, J = 7.1, 1.1 Hz, 1H), 7.46 (dt, J = 9.1, 1.1 Hz, 1H), 7.07 (ddd, J = 9.1, 6.6, 1.3 Hz, 1H), 6.73–6.68 (m, 2H), 2.70–2.55 (m, 1H), 2.57–2.46 (m, 1H), 2.46–2.35 (m, 2H), 1.84 (d, J = 8.5 Hz, 3H), 1.70–1.67 (m, 1H), 1.67–1.63 (m, 1H), 1.62–1.59 (m, 1H), 1.52–1.49 (m, 1H), 1.48–1.46 (m, 1H), 1.39–1.35 (m, 1H), 1.29–1.26 (m, 1H), 1.21–1.19 (m, 1H), 1.18 (s, 9H), 1.09 (s, 6H), 1.06 (d, J = 7.0 Hz, 3H), 0.95 (s, 3H). 13C-NMR (100 MHz, CDCl3): δ 149.1, 148.0, 142.2, 131.2, 124.5, 123.8, 123.5, 123.3, 116.8, 110.9, 107.4, 56.5, 41.1, 38.3, 37.6, 36.3, 34.3, 32.4, 31.5, 30.1, 29.6, 28.0, 25.6, 24.7, 24.3, 24.0, 22.4, 18.2, 17.5, 17.1. HRMS (ESI+): m/z: Calcd. for C31H43N3O2 [M + H]+: 489.3355; found: 490.3407.
3. Results and Discussion
We began by isolating 5α-hydroxyvouacapane 5 from the dichloromethane extract of C. pulcherrima stem through column chromatography using mixtures of hexanes–EtOAC (9:1 v/v). This process yielded 96 mg (0.02% yield) of 5α-hydroxyvouacapane 5 as colorless crystals from 400 g of the stem material. Spectroscopic characterization agreed with the data reported by Yodsaoue et al. [9]. Subsequently, 5α-hydroxyvouacapane 5 was subjected to a Vilsmeier–Haack formylation, resulting in the formation of vouacapane-aldehyde 6 in 37% yield (Scheme 1), which served as the starting material for the GBB reaction. 1D-NMR techniques (1H and 13C) confirmed the presence of the aldehyde proton at δ 9.47 and the carbonyl group at δ 176.8. The yield obtained for this compound was lower than that reported by Servin et al. under the same formylation conditions [16]. This variation can be attributed to the fact that the starting material for compound 5 contains a hydroxyl group, which may react with POCl3, causing an alcohol dehydration reaction that leads to the formation of alkenes as byproducts.

Scheme 1.
Formylation of 5α-hydroxyvouacapane via the Vilsmeier–Haack method.
Thus, aiming to evaluate the synthetic potential of vouacapane-aldehyde 6, a preliminary assessment of the reaction GBB by using as model reaction formyl-5α-hydroxyvouacapane 6, 2-aminopyrimidine 7, and tert-butyl isocyanide 8 under the conditions recently reported by our research group [13] yielded imidazo-5α-hydroxyvouacapane 9 in 74% yield (Scheme 2).
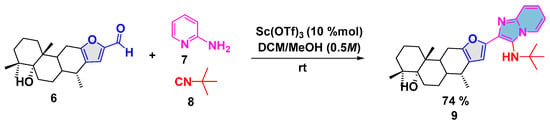
Scheme 2.
Synthesis of imidazo-5α-hydroxyvouacapane 9 via the GBB reaction.
The structure of imidazo-5α-hydroxyvouacapane 9 was elucidated via 1H NMR, 13C NMR, and HRMS. The inspection of the 1H NMR spectroscopic data revealed four new aromatic proton resonances that belong to the fused imidazole. Thus, doublets of triplets signal at δ 8.20 (dt, J = 6.9, 1.2 Hz, 1H) and δ 7.46 (dt, J = 9.1, 1.2 Hz, 1H) were observed, as well as a doublet of doublets of doublets signal at δ 7.07 (ddd, J = 9.1, 6.6, 1.2 Hz, 1H). One signal overlapped with the proton resonance from the furan ring between δ 6.73 and 6.68 was observed, while a singlet signal at δ 1.18 was assigned to the tert-butyl group from the isocyanide moiety. The 13C NMR spectrum revealed a signal at δ 123.3 assigned to the carbon bound to the furan ring. At δ 142.2, a signal from the carbon bridgehead of the fused system was observed, and at δ 149.1, the signal corresponds to C-N from the isocyanide group.
4. Conclusions
In summary, we have demonstrated the initial utility of the GBB multicomponent reaction as a potent synthetic tool for the semi-synthesis of a novel heterocyclic system, imidazo-5α-hydroxyvouacapane 9. This compound was efficiently obtained with good yield, showcasing the rapid and straightforward nature of the synthesis. This work represents a valuable contribution to the field of natural products and the realm of multicomponent reactions.
Author Contributions
Conceptualization, C.J.C.-G. and R.E.d.R.; methodology, G.S.-G. and A.A.F.-L.; software, L.C.-G.; validation, G.R.-G.; formal analysis, L.C.-G.; investigation, C.J.C.-G. and M.A.G.-H.; resources, C.J.C.-G. and R.E.d.R.; data curation, G.S.-G. and A.A.F.-L.; writing—original draft preparation, C.J.C.-G. and G.S.-G.; writing—review and editing, C.J.C.-G. and L.C.-G.; visualization, A.A.F.-L. and G.R.-G.; supervision, C.J.C.-G.; project administration, C.J.C.-G. and L.C.-G.; funding acquisition, C.J.C.-G. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledged the financial support from Programa para el Desarrollo Profesional Docente, para el Tipo Superior (PRODEP PTC-404), and CIC-UMSNH (14766).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Carlos C.-G. is grateful for financial support from Programa para el Desarrollo Profesional Docente, para el Tipo Superior (PRODEP PTC-404), and CIC-UMSNH (14766). Gabriela S.-G. is grateful to CONACYT for scholarships 772950.
Conflicts of Interest
The authors declare no conflicts of interest or state.
References
- Hong, J. Role of natural product diversity in chemical biology. Curr. Opin. Chem. Biol. 2011, 15, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Majhi, S.; Das, D. Chemical derivatization of natural products: Semisynthesis and pharmacological aspects—A decade update. Tetrahedron 2021, 78, 131801. [Google Scholar] [CrossRef]
- Vollmann, D.J.; Winand, L.; Nett, M. Emerging concepts in the semisynthetic and mutasynthetic production of natural products. Curr. Opin. Biotechnol. 2022, 77, 102761. [Google Scholar] [CrossRef] [PubMed]
- Zhi, S.; Ma, X.; Zhang, W. Consecutive multicomponent reactions for the synthesis of complex molecules. Org. Biomol. Chem. 2019, 17, 7632–7650. [Google Scholar] [CrossRef] [PubMed]
- Graebin, C.S.; Ribeiro, F.V.; Rogério, K.R.; Kummerle, A.E. Multicomponent reactions for the synthesis of bioactive compounds: A review. Curr. Org. Chem. 2019, 16, 855–899. [Google Scholar] [CrossRef] [PubMed]
- Yodsaoue, O.; Karalai, C.; Ponglimanont, C.; Tewtrakul, S.; Chantrapromma, S. Pulcherrins D-R, potential anti-inflammatory diterpenoids from the roots of Caesalpinia pulcherrima. Tetrahedron 2011, 67, 6838–6846. [Google Scholar] [CrossRef]
- McPherson, D.D.; Che, C.-T.; Cordell, G.A.; Soejarto, D.D.; Pezzuto, J.M.; Fong, H.H.S. Diterpenoids from Caesalpinia pulcherrima. Phytochemistry 1986, 25, 167–170. [Google Scholar] [CrossRef]
- Promsawan, N.; Kittakoop, P.; Boonphong, S.; Nongkunsarn, P. Antitubercular cassane furanditerpenoids from the roots of Caesalpinia pulcherrima. Planta Med. 2003, 69, 776–777. [Google Scholar] [PubMed]
- Ragasa, C.Y.; Hofilena, J.G.; Rideout, J.A. New furanoid diterpenes from Caesapinia pulcherrima. J. Nat. Prod. 2002, 65, 1107. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.D.; Freyer, A.J.; Webb, R.L.; Zuber, G.; Reichwein, R.; Bean, M.F.; Faucette, L.; Johnson, R.K. Pulcherrimins A–D, Novel dibenzoates from Caesalpinia pulcherrima with selective activity against DNA-Repair-deficient yeast mutants. Tetrahedron 1997, 53, 1583–1592. [Google Scholar] [CrossRef]
- Niño-Pantoja, I.; Gallardo-Alfonzo, A.; Solis-Santos, M.; Ordoñez, M.; Contreras-Celedón, C.; Islas-Jácome, A.; Chacón-García, L.; Cortés-García, C.J. Synthesis of 1,5-disubstituted tetrazole−indolizine bis-heterocycles and their copper (II) recognizing properties. Eur. J. Org. Chem. 2022, 34, 8–17. [Google Scholar] [CrossRef]
- Aguilar-Morales, C.; Servín-García, G.; Río, R.; Islas-Jácome, A.; Gámez-Montaño, R.; Chacón-García, L.; Cortés-García, C. Synthesis of novel hybrid 1,5-disusbtituted 1H-tetrazol-5yl 4,5-dihydro [1,2,3]triazolo [1,5-a]pyrazin-6-ones via high-order MCR-SN2/intramolecular [3+2] cycloaddition sequence. Synth. Commun. 2022, 53, 127–134. [Google Scholar] [CrossRef]
- Aguilar-Morales, C.M.; Araujo-Huitrado, J.G.; López-Hernández, V.; Contreras-Celedón, C.; Islas-Jácome, A.; Granados-López, A.J.; Solorio-Alvarado, C.R.; Adrián-López, V.; Chacón-García, V.; Cortés-García, C.J. A one-pot six-component reaction for the synthesis of 1,5-disubstituted tetrazol-1,2,3-triazole hybrids and their cytotoxic activity against the MCF-7 Cell Line. Molecules 2021, 6, 6104. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Morales, C.M.; de Loera, D.; Contreras-Celedón, C.; Cortés-García, C.J.; Chacón-García, L. Synthesis of 1,5-disubstituted tetrazole-1,2,3 triazoles hybrids via Ugi-azide/CuAAC. Synth. Commun. 2019, 49, 2086–2095. [Google Scholar] [CrossRef]
- Servín-García, G.; Chacón-García, L.; Islás-Jácome, A.; Gómez-Hurtado, M.A.; Rodríguez-García, G.; del Río, R.E.; Cortés-García, C.J. Late stage functionalization of vouacapane derivatives from Caesalpinia platyloba by a Groebke−Blackburn−Bienaymé reaction. J. Heterocycl. Chem 2023. early view. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).