Innovative Approaches in Acyl Sonogashira Coupling: Impact of Supported CuNPs and Cu-PdNPs Nanocatalysts †
Abstract
:1. Introduction
2. Experimental Procedures
2.1. General Methods
2.2. Preparation of Catalysts
2.2.1. Preparation of CuNPs Catalysts
2.2.2. Preparation of Cu-Pd Catalysts
2.3. General Procedure for Acyl Sonogashira Reactions
2.4. General Procedure for Sonogashira Reactions
3. Results and Discussion
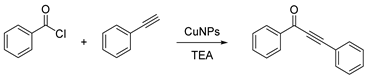
3.1. CuNPs Catalyzed Acyl Sonogashira Coupling
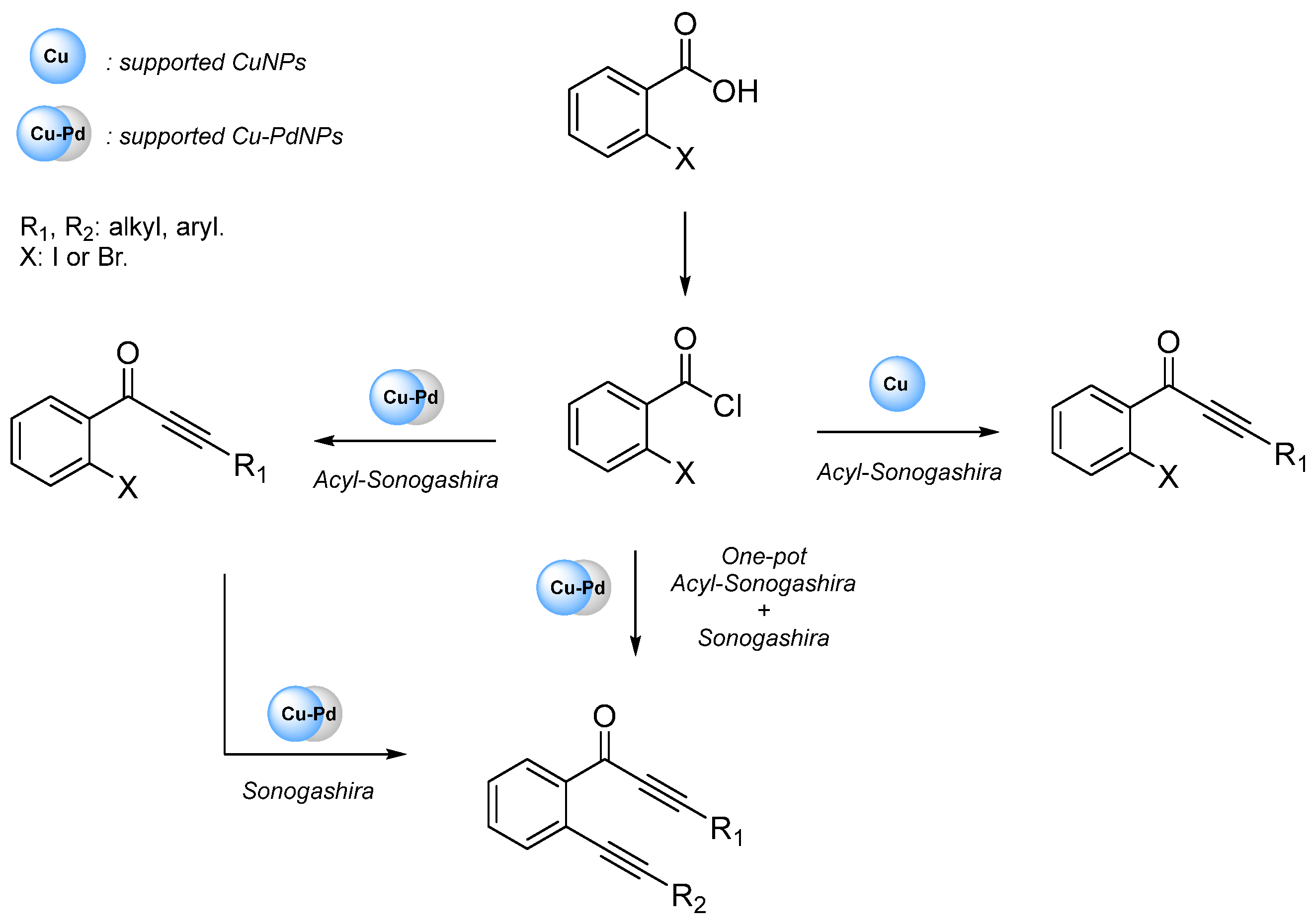
3.2. Cu-PdNPs-Catalyzed Acyl Sonogashira Coupling
3.3. Cu-PdNPs-Catalyzed Sonogashira Coupling of Aryl Iodides and Terminal Alkynes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, W.; Wang, Y.; Wu, X.; Yao, X. Palladium-, Ligand-, and Solvent-Free Synthesis of Ynones by the Coupling of Acyl Chlorides and Terminal Alkynes in the Presence of a Reusable Copper Nanoparticle Catalyst. Green Chem. 2013, 15, 2356–2360. [Google Scholar] [CrossRef]
- Albano, G.; Aronica, L.A. Acyl Sonogashira Cross-Coupling: State of the Art and Application to the Synthesis of Heterocyclic Compounds. Catalysts 2020, 10, 25. [Google Scholar] [CrossRef]
- Wu, X.-F.; Neumann, H.; Beller, M. Palladium-Catalyzed Carbonylative Coupling Reactions between Ar–X and Carbon Nucleophiles. Chem. Soc. Rev. 2011, 40, 4986–5009. [Google Scholar] [CrossRef] [PubMed]
- Tohda, K.; Hagihara, N.Y.S. A Convenient Synthesis of 1-Alkynyl Ketones and 2-Alkynamides. Synthesis 1977, 1977, 777–778. [Google Scholar] [CrossRef]
- Cox, R.J.; Ritson, D.J.; Dane, T.A.; Berge, J.; Charmant, J.P.H.; Kantacha, A. Room Temperature Palladium Catalysed Coupling of Acyl Chlorides with Terminal Alkynes. Chem. Commun. 2005, 8, 1037–1039. [Google Scholar] [CrossRef]
- Alonso, F.; Moglie, Y.; Radivoy, G. Copper Nanoparticles in Click Chemistry. Acc. Chem. Res. 2015, 48, 2516–2528. [Google Scholar] [CrossRef]
- Buxaderas, E.; Graziano-Mayer, M.; Volpe, M.A.; Radivoy, G. Bimetallic Cu-Pd Nanoparticles Supported on Bio-Silica as an Efficient Catalyst for Selective Aerobic Oxidation of Benzylic Alcohols. Synthesis 2017, 49, 1387–1393. [Google Scholar] [CrossRef]
- Stabile, S.A.; Bjerg, E.; Radivoy, G.E. Copper Nanoparticles on Montmorillonite K-10: A Versatile Catalyst for the One-Pot Synthesis of 3,5-Disubstituted Isoxazoles Using Various Methodologies. Synthesis, 2023; accepted. [Google Scholar]
- Nador, F.; Volpe, M.A.; Alonso, F.; Feldhoff, A.; Kirschning, A.; Radivoy, G. Copper Nanoparticles Supported on Silica Coated Maghemite as Versatile, Magnetically Recoverable and Reusable Catalyst for Alkyne Coupling and Cycloaddition Reactions. Appl. Catal. A Gen. 2013, 455, 39–45. [Google Scholar] [CrossRef]
- Wang, D.; Astruc, D. Fast-Growing Field of Magnetically Recyclable Nanocatalysts. Chem. Rev. 2014, 114, 6949–6985. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, F.; Oliva, A.I.; Pericas, M.A. Direct Copper(I)-Catalyzed Cycloaddition of Organic Azides with TMS-Protected Alkynes. Synlett 2010, 2010, 1873–1877. [Google Scholar] [CrossRef]

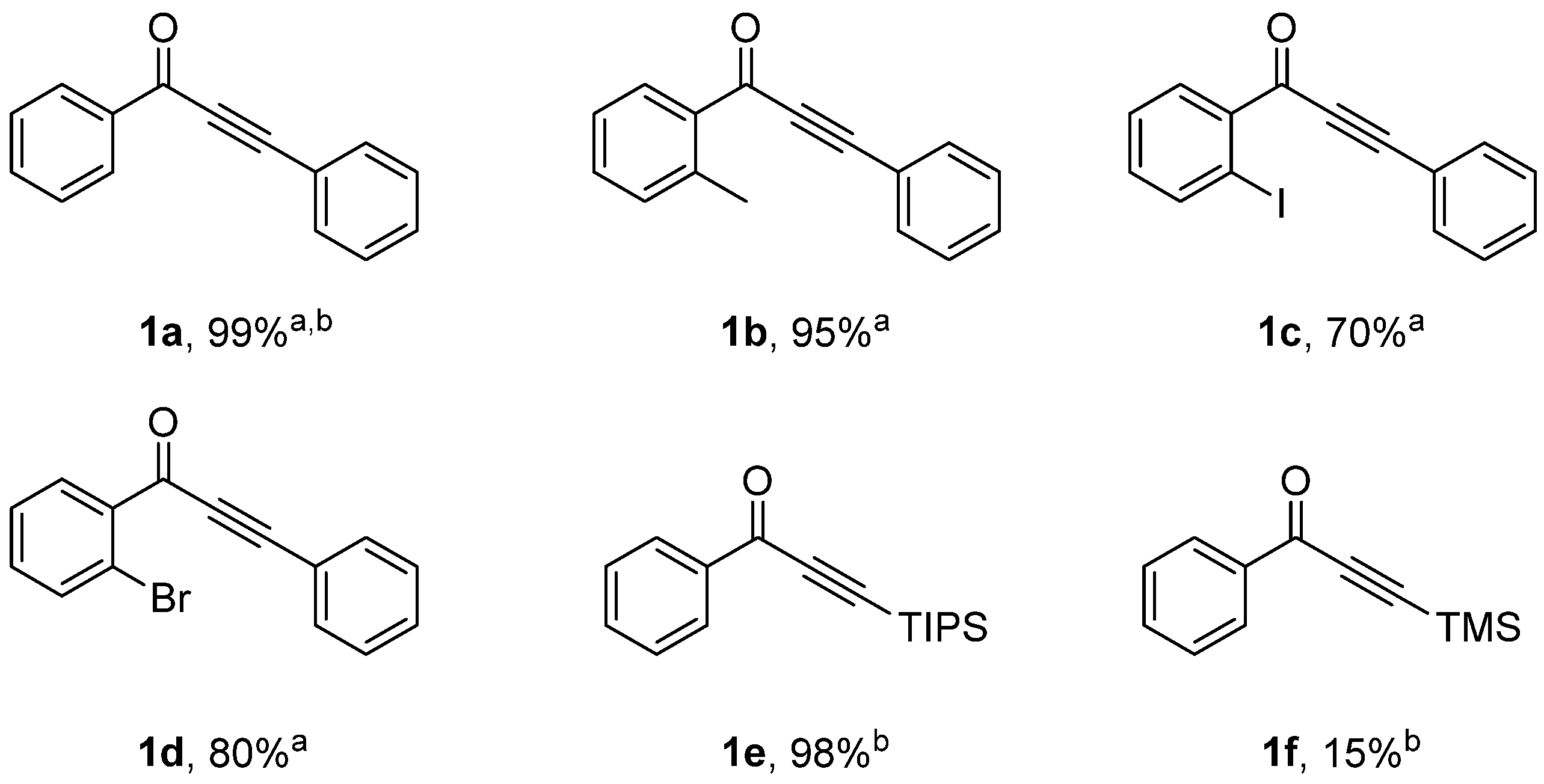
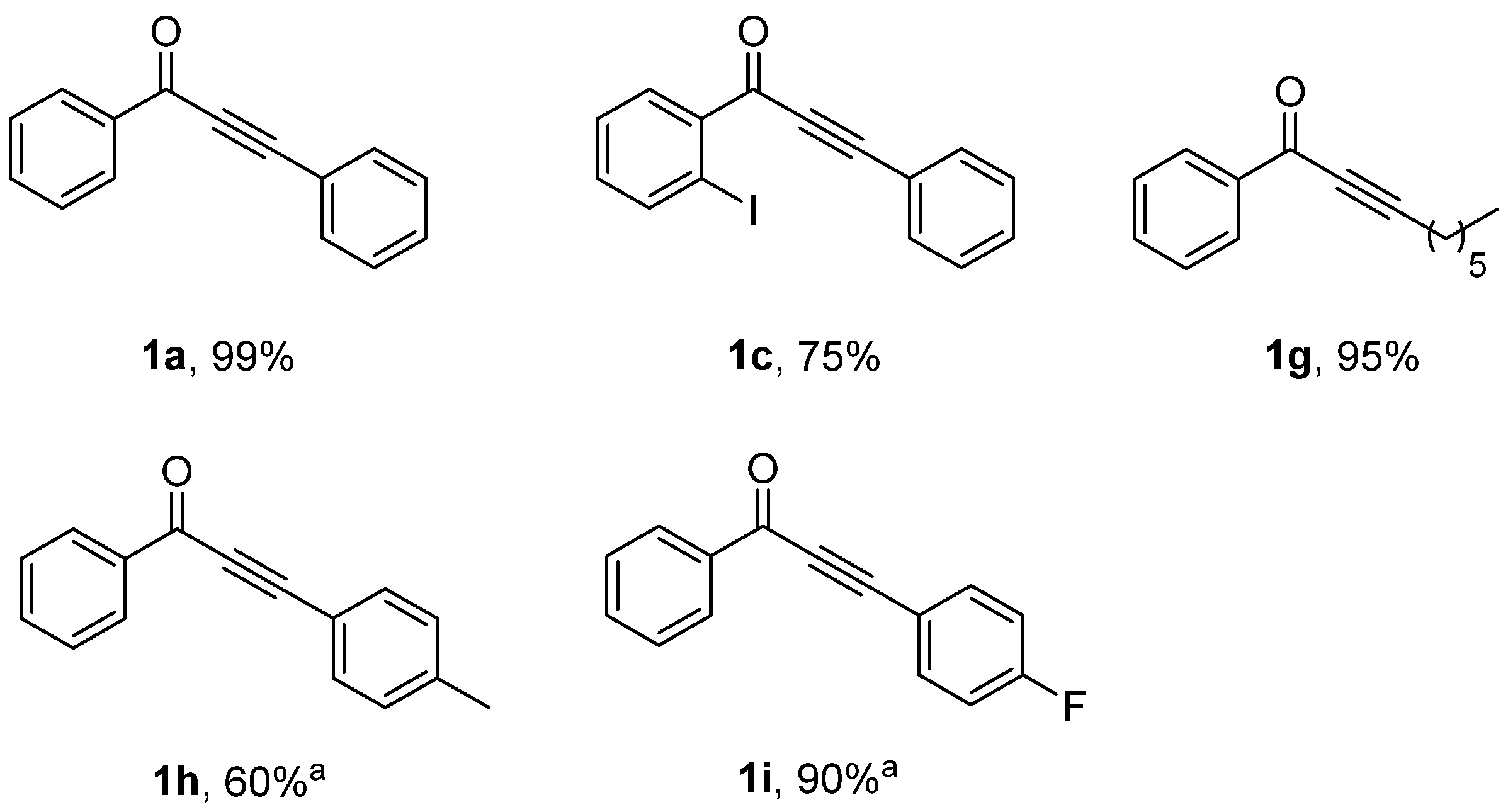
| Entry | CuNPs/MK-10 (mg) | T (°C) | T (h) | Yield% b, c |
|---|---|---|---|---|
| 1 | 5 | 60 | 20 | 59 |
| 2 | 10 | 60 | 20 | 88 (100) |
| 3 | 20 | 60 | 20 | 71 |
| 4 | 10 | 20 | 20 | 42 (45) |
| 5 | 10 | 40 | 20 | 71 (82) |
| 6 | 10 | 60 | 4 | (81) |
| 7 | 10 | 60 | 8 | (92) |
| 8 | 10 | 80 | 4 | (100) |
| 9 | 10 d | 80 | 4 | (99) |
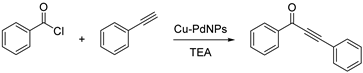
| Entry | Cu-PdNPs/Celite (mg) | T (°C) | T (h) | Yield% b |
|---|---|---|---|---|
| 1 | 5 | r.t | 2 | 40 |
| 2 | 10 | r.t | 2 | 70 |
| 3 | 5 | r.t | 4 | 99 |
| 4 | 5 | r.t | 4 | 83 c |
| 5 | 10 | r.t | 4 | 85 c |
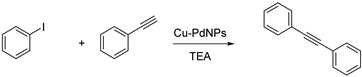
| Entry | Cu-PdNPs/Celite (mg) | T (°C) | T (h) | Yield% b |
|---|---|---|---|---|
| 1 | 5 | 80 | 4 | 65 |
| 2 | 10 | 80 | 4 | 76 |
| 3 | 5 | 80 | 6 | 82 |
| 4 | 5 | 80 | 6 | 84 c |
| 5 | 10 | 80 | 6 | 88 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stabile, S.; Radivoy, G. Innovative Approaches in Acyl Sonogashira Coupling: Impact of Supported CuNPs and Cu-PdNPs Nanocatalysts. Chem. Proc. 2023, 14, 76. https://doi.org/10.3390/ecsoc-27-16090
Stabile S, Radivoy G. Innovative Approaches in Acyl Sonogashira Coupling: Impact of Supported CuNPs and Cu-PdNPs Nanocatalysts. Chemistry Proceedings. 2023; 14(1):76. https://doi.org/10.3390/ecsoc-27-16090
Chicago/Turabian StyleStabile, Santiago, and Gabriel Radivoy. 2023. "Innovative Approaches in Acyl Sonogashira Coupling: Impact of Supported CuNPs and Cu-PdNPs Nanocatalysts" Chemistry Proceedings 14, no. 1: 76. https://doi.org/10.3390/ecsoc-27-16090
APA StyleStabile, S., & Radivoy, G. (2023). Innovative Approaches in Acyl Sonogashira Coupling: Impact of Supported CuNPs and Cu-PdNPs Nanocatalysts. Chemistry Proceedings, 14(1), 76. https://doi.org/10.3390/ecsoc-27-16090






