Synthesis of Symmetrical Monocarbonyl Analogs of Curcumin Containing a 2-Bromobenzylidene Moiety and Spectrophotometric Assessment of Their Reactivity with 2-(Dimethylamino)ethanthiol †
Abstract
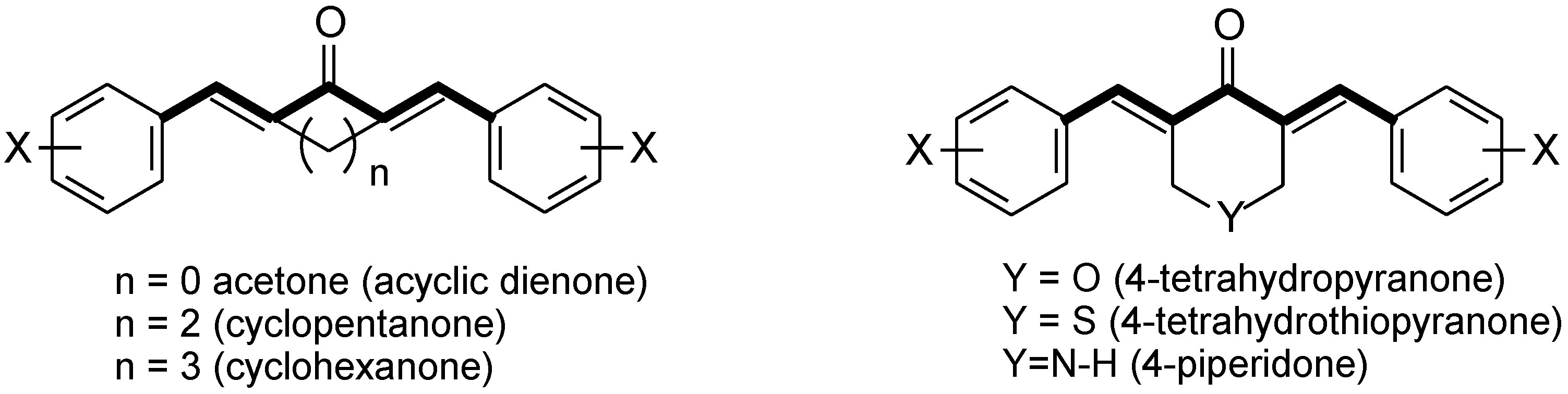
1. Introduction
2. Materials and Methods
2.1. General
2.2. Preparation of 4tB2BrCX
2.3. UV/Vis Kinetic Thiol Assay
3. Results and Discussion
3.1. Chemistry
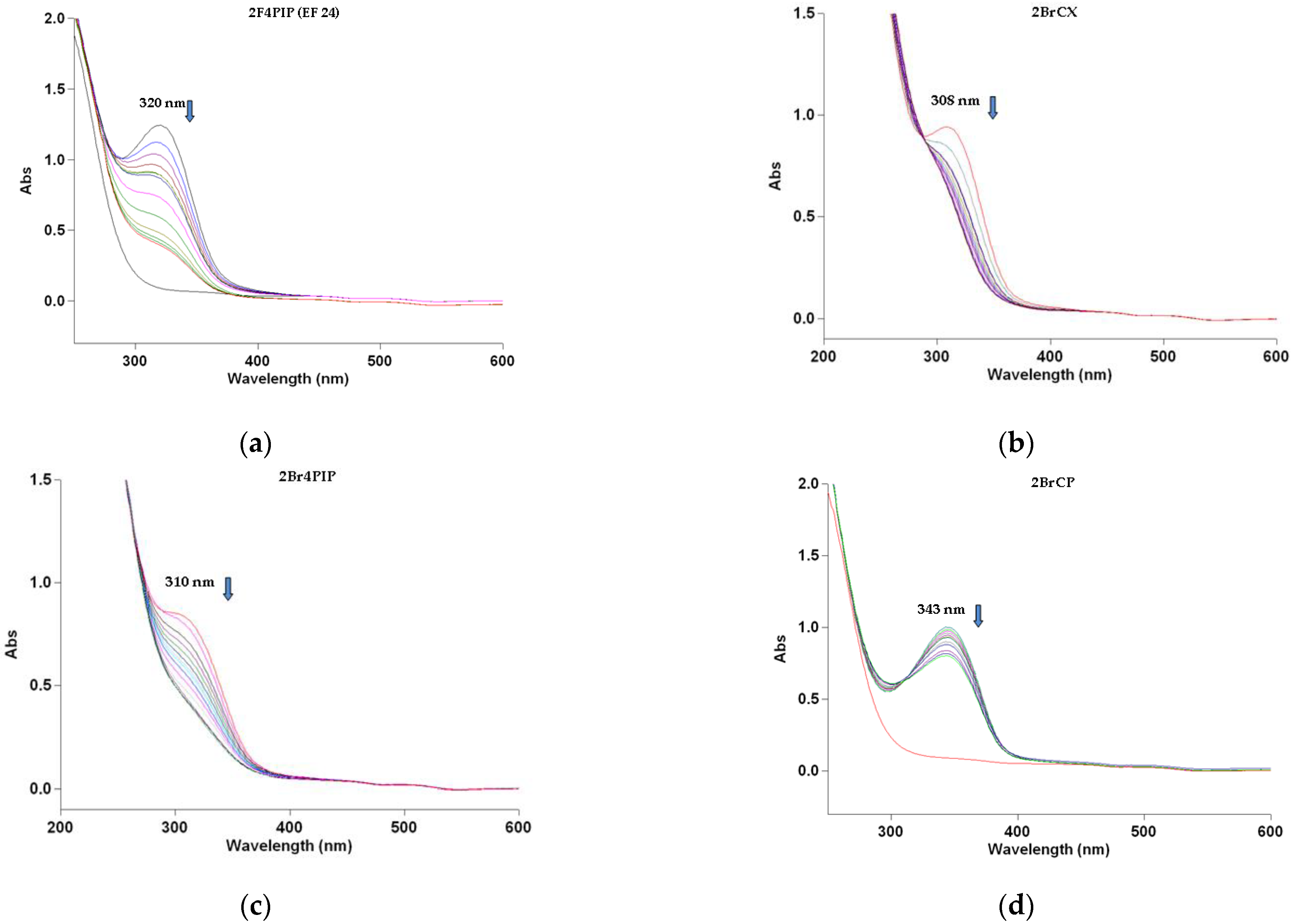
3.2. Spectrophotometric Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Das, U.; Sharma, R.K.; Dimmock, J.R. 1,5-Diaryl-3-Oxo-1,4-Pentadienes: A Case for Antineoplastics with Multiple Targets. Curr. Med. Chem. 2009, 16, 2001–2020. [Google Scholar] [CrossRef]
- Hossain, M.; Das, U.; Dimmock, J.R. Recent Advances in α,β-Unsaturated Carbonyl Compounds as Mitochondrial Toxins. Eur. J. Med. Chem. 2019, 183, 111687. [Google Scholar] [CrossRef] [PubMed]
- Bazzaro, M.; Linder, S. Dienone Compounds: Targets and Pharmacological Responses. J. Med. Chem. 2020, 63, 15075–15093. [Google Scholar] [CrossRef] [PubMed]
- Moreira, J.; Saraiva, L.; Pinto, M.M.; Cidade, H. Diarylpentanoids with Antitumor Activity: A Critical Review of Structure-Activity Relationship Studies. Eur. J. Med. Chem. 2020, 192, 112177. [Google Scholar] [CrossRef] [PubMed]
- Moreira, J.; Saraiva, L.; Pinto, M.M.; Cidade, H. Bioactive Diarylpentanoids: Insights into the Biological Effects beyond Antitumor Activity and Structure–Activity Relationships. Molecules 2022, 27, 6340. [Google Scholar] [CrossRef] [PubMed]
- Yamakoshi, H.; Ohori, H.; Kudo, C.; Sato, A.; Kanoh, N.; Ishioka, C.; Shibata, H.; Iwabuchi, Y. Structure–Activity Relationship of C5-Curcuminoids and Synthesis of Their Molecular Probes Thereof. Bioorg. Med. Chem. 2010, 18, 1083–1092. [Google Scholar] [CrossRef]
- Vatsadze, S.Z.; Golikov, A.G.; Kriven’ko, A.P.; Zyk, N.V. Chemistry of Cross-Conjugated Dienones. Russ. Chem. Rev. 2008, 77, 661. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, Z.; Liang, G. Promising Curcumin-Based Drug Design: Mono-Carbonyl Analogues of Curcumin (MACs). Curr. Pharm. Des. 2013, 19, 2114–2135. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. Chemical and Structural Features Influencing the Biological Activity of Curcumin. Curr. Pharm. Des. 2013, 19, 2093–2100. [Google Scholar] [CrossRef]
- Shetty, D.; Kim, Y.J.; Shim, H.; Snyder, J.P. Eliminating the Heart from the Curcumin Molecule: Monocarbonyl Curcumin Mimics (MACs). Molecules 2014, 20, 249–292. [Google Scholar] [CrossRef]
- Bairwa, K.; Grover, J.; Kania, M.; Jachak, S.M. Recent Developments in Chemistry and Biology of Curcumin Analogues. RSC Adv. 2014, 4, 13946–13978. [Google Scholar] [CrossRef]
- Chainoglou, E.; Hadjipavlou-Litina, D. Curcumin Analogues and Derivatives with Anti-Proliferative and Anti-Inflammatory Activity: Structural Characteristics and Molecular Targets. Expert Opin. Drug Discov. 2019, 14, 821–842. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, S.V.; Kuthe, P.; Patil, S.M.; Nagras, O.; Sarkate, A.P. A Review: Exploring Synthetic Schemes and Structure-Activity Relationship (SAR) Studies of Mono-Carbonyl Curcumin Analogues for Cytotoxicity Inhibitory Anticancer Activity. Curr. Org. Synth. 2023, 20, 821–837. [Google Scholar] [CrossRef] [PubMed]
- Das, U.; Doroudi, A.; Das, S.; Bandy, B.; Balzarini, J.; De Clercq, E.; Dimmock, J.R. E,E-2-Benzylidene-6-(Nitrobenzylidene)Cyclohexanones: Syntheses, Cytotoxicity and an Examination of Some of Their Electronic, Steric, and Hydrophobic Properties. Bioorg. Med. Chem. 2008, 16, 6261–6268. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Adams, B.K.; Ferstl, E.M.; Davis, M.C.; Herold, M.; Kurtkaya, S.; Camalier, R.F.; Hollingshead, M.G.; Kaur, G.; Sausville, E.A.; Rickles, F.R.; et al. Synthesis and Biological Evaluation of Novel Curcumin Analogs as Anti-Cancer and Anti-Angiogenesis Agents. Bioorg. Med. Chem. 2004, 12, 3871–3883. [Google Scholar] [CrossRef] [PubMed]
- Adams, B.K.; Cai, J.; Armstrong, J.; Herold, M.; Lu, Y.J.; Sun, A.; Snyder, J.P.; Liotta, D.C.; Jones, D.P.; Shoji, M. EF24, a Novel Synthetic Curcumin Analog, Induces Apoptosis in Cancer Cells via a Redox-Dependent Mechanism. Anticancer Drugs 2005, 16, 263–275. [Google Scholar] [CrossRef]
- Liang, G.; Zhou, H.; Wang, Y.; Gurley, E.C.; Feng, B.; Chen, L.; Xiao, J.; Yang, S.; Li, X. Inhibition of LPS-Induced Production of Inflammatory Factors in the Macrophages by Mono-Carbonyl Analogues of Curcumin. J. Cell. Mol. Med. 2009, 13, 3370–3379. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Shao, L.; Wang, Y.; Zhao, C.; Chu, Y.; Xiao, J.; Zhao, Y.; Li, X.; Yang, S. Exploration and Synthesis of Curcumin Analogues with Improved Structural Stability Both in Vitro and in Vivo as Cytotoxic Agents. Bioorg. Med. Chem. 2009, 17, 2623–2631. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, Y.; Cai, L.; Cai, Y.; Hu, J.; Yu, C.; Li, J.; Feng, Z.; Yang, S.; Li, X.; et al. Inhibition of High Glucose-Induced Inflammatory Response and Macrophage Infiltration by a Novel Curcumin Derivative Prevents Renal Injury in Diabetic Rats. Br. J. Pharmacol. 2012, 166, 1169–1182. [Google Scholar] [CrossRef]
- Mladenov, M.; Bogdanov, J.; Bogdanov, B.; Hadzi-Petrushev, N.; Kamkin, A.; Stojchevski, R.; Avtanski, D. Efficacy of the Monocarbonyl Curcumin Analog C66 in the Reduction of Diabetes-Associated Cardiovascular and Kidney Complications. Mol. Med. 2022, 28, 129. [Google Scholar] [CrossRef]
- Qian, Y.; Zhong, P.; Liang, D.; Xu, Z.; Skibba, M.; Zeng, C.; Li, X.; Wei, T.; Wu, L.; Liang, G. A Newly Designed Curcumin Analog Y20 Mitigates Cardiac Injury via Anti-Inflammatory and Anti-Oxidant Actions in Obese Rats. PLoS ONE 2015, 10, e0120215. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Yang, S.; Jiang, L.; Zhao, Y.; Shao, L.; Xiao, J.; Ye, F.; Li, Y.; Li, X. Synthesis and Anti-Bacterial Properties of Mono-Carbonyl Analogues of Curcumin. Chem. Pharm. Bull. 2008, 56, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Fioravanti, R.; Tomassi, S.; Di Bello, E.; Romanelli, A.; Plateroti, A.M.; Benedetti, R.; Conte, M.; Novellino, E.; Altucci, L.; Valente, S.; et al. Properly Substituted Cyclic Bis-(2-Bromobenzylidene) Compounds Behaved as Dual P300/CARM1 Inhibitors and Induced Apoptosis in Cancer Cells. Molecules 2020, 25, 3122. [Google Scholar] [CrossRef] [PubMed]
- Todorovska, I.; Dragarska, K.; Bogdanov, J. A Combined 2D- and 3D-QSAR Study, Design and Synthesis of Some Monocarbonyl Curcumin Analogs as Potential Inhibitors of MDA-MB-231 Breast Cancer Cells. Chem. Proc. 2022, 12, 5. [Google Scholar] [CrossRef]
- Lozanovski, Z.; Petreska-Stanoeva, J.; Bogdanov, J. Development of a Spectrophotometric Method for Assessment of the Relative Reactivity of Monocarbonyl Analogs of Curcumin with 2-(Dimethylamino)Ethanethiol. Maced. J. Chem. Chem. Eng. 2023, 42, 13–24. [Google Scholar] [CrossRef]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef]
- Baell, J.; Walters, M.A. Chemistry: Chemical Con Artists Foil Drug Discovery. Nature 2014, 513, 481–483. [Google Scholar] [CrossRef]
- Hadzi-Petrushev, N.; Bogdanov, J.; Krajoska, J.; Ilievska, J.; Bogdanova-Popov, B.; Gjorgievska, E.; Mitrokhin, V.; Sopi, R.; Gagov, H.; Kamkin, A.; et al. Comparative Study of the Antioxidant Properties of Monocarbonyl Curcumin Analogues C66 and B2BrBC in Isoproteranol Induced Cardiac Damage. Life Sci. 2018, 197, 10–18. [Google Scholar] [CrossRef]
- Stamenkovska, M.; Thaçi, Q.; Hadzi-Petrushev, N.; Angelovski, M.; Bogdanov, J.; Reçica, S.; Kryeziu, I.; Gagov, H.; Mitrokhin, V.; Kamkin, A.; et al. Curcumin Analogs (B2BrBC and C66) Supplementation Attenuates Airway Hyperreactivity and Promote Airway Relaxation in Neonatal Rats Exposed to Hyperoxia. Physiol. Rep. 2020, 8, e14555. [Google Scholar] [CrossRef]
- Sun, A.; Lu, Y.J.; Hu, H.; Shoji, M.; Liotta, D.C.; Snyder, J.P. Curcumin Analog Cytotoxicity against Breast Cancer Cells: Exploitation of a Redox-Dependent Mechanism. Bioorg. Med. Chem. Lett. 2009, 19, 6627–6631. [Google Scholar] [CrossRef]
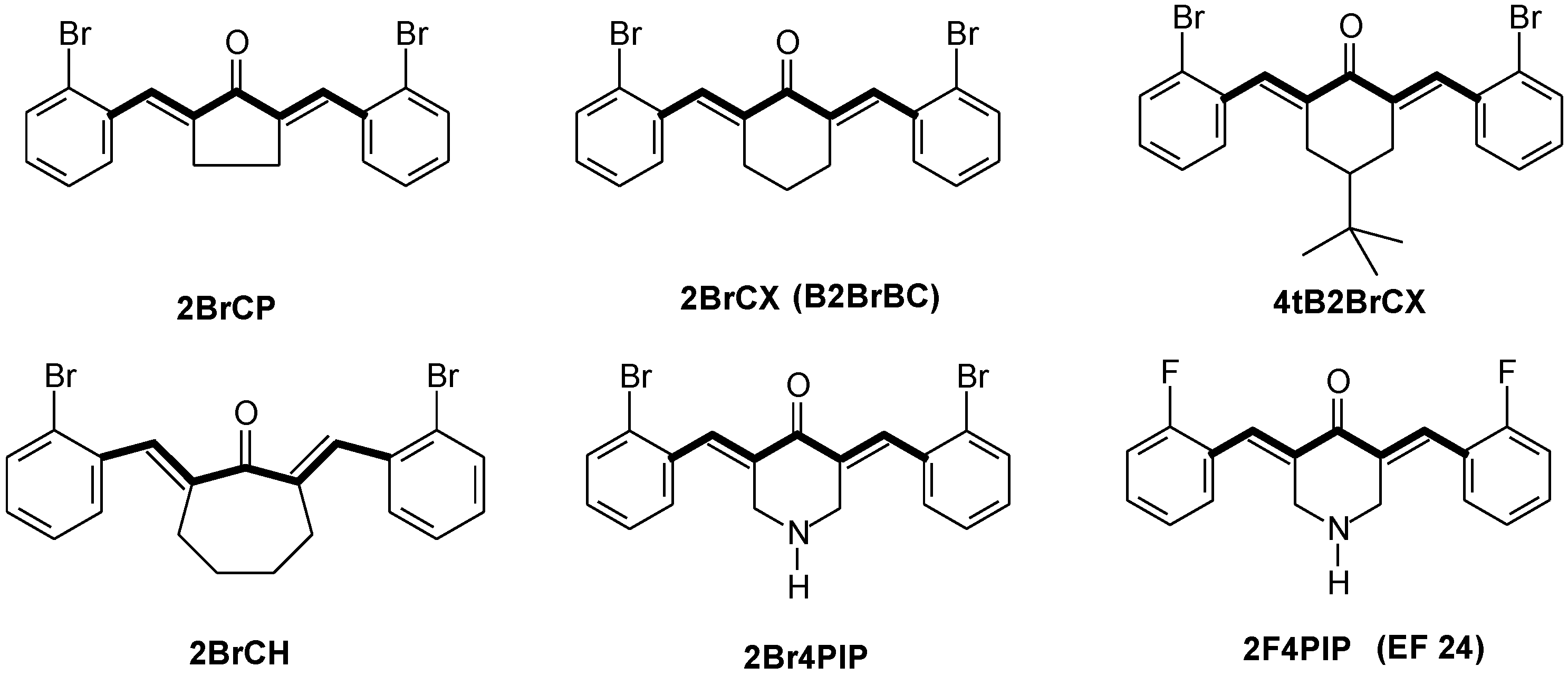
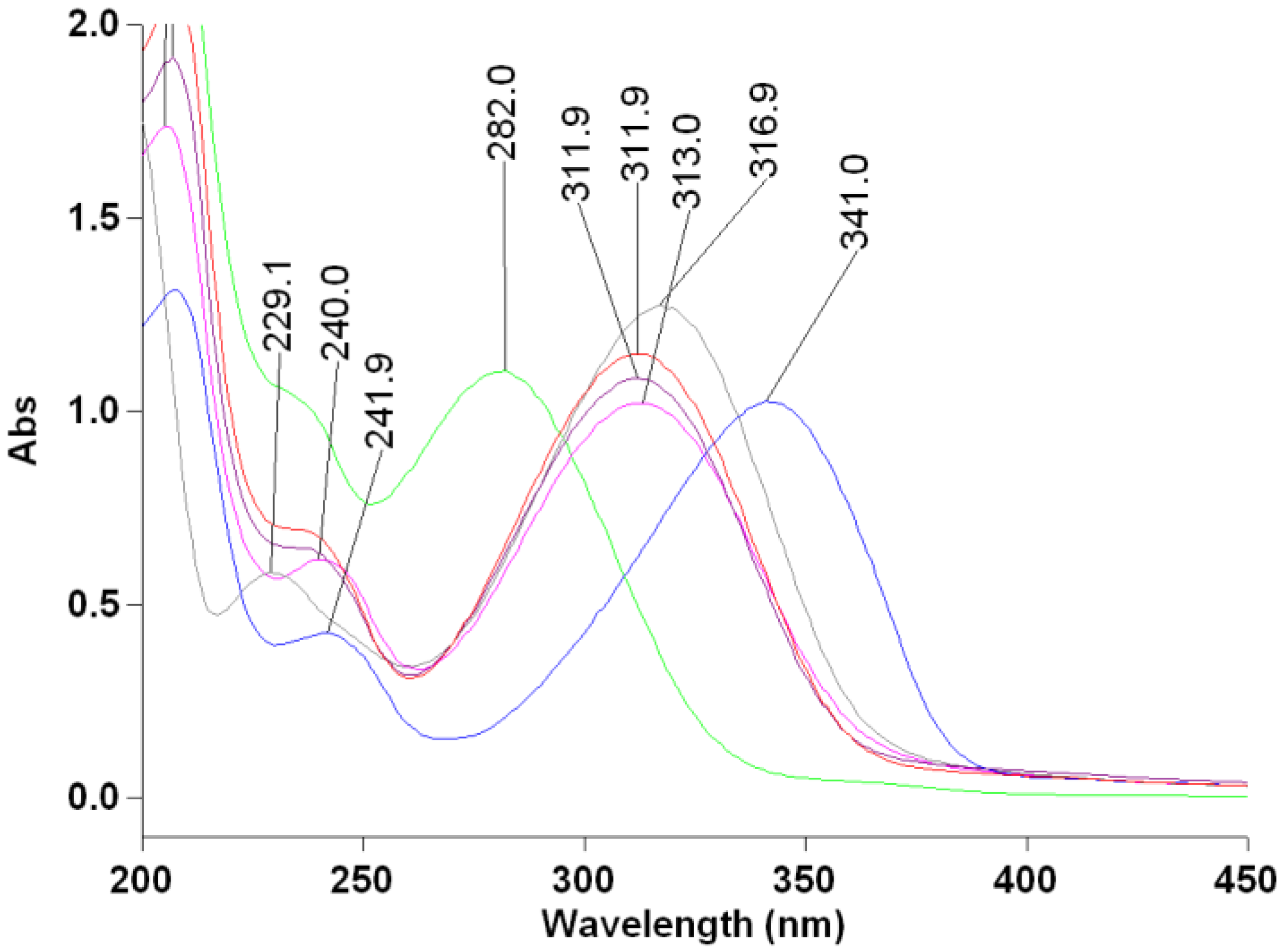
| Comp. | mp (°C) | FT-IR (cm−1) | UV-VIS λmax1 (nm) | UV-VIS λmax2 (nm) | GC-MS tR (min) | EI-MS (m/z) |
|---|---|---|---|---|---|---|
| 2BrCP | 165–166 | 1693 (C=O) | 341 | 242 nm | 22.702 | M+ + 4 (420), M+ + 2 (418), M+ (416) |
| 2BrCX | 131–133 | 1662 (C=O) | 312 nm | 237 nm | 21.206 | M+ + 4 (434), M+ + 2 (432), M+ (430) |
| 4tB2BrCX | 159–161 | 1670 (C=O) | 312 nm | 236 nm | 23.176 | M+ + 4 (490), M+ + 2 (488), M+ (486) |
| 2BrCH | 109–112 | 1664 (C=O) | 282 nm | 235 nm | 21.437 | M+ + 4 (448), M+ + 2 (446), M+ (444) |
| 2Br4PIP | 162–163 | 1669 (C=O) | 313 nm | 240 nm | 24.775 | M+ + 4 (435), M+ + 2 (433), M+ (431)+ |
| EF 24 | 134–136 | 1660 (C=O) | 317 nm | 229 nm | 24.316 | M+ (311) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozanovski, Z.; Todorovska, I.; Dragarska, K.; Bogdanov, J. Synthesis of Symmetrical Monocarbonyl Analogs of Curcumin Containing a 2-Bromobenzylidene Moiety and Spectrophotometric Assessment of Their Reactivity with 2-(Dimethylamino)ethanthiol. Chem. Proc. 2023, 14, 7. https://doi.org/10.3390/ecsoc-27-16084
Lozanovski Z, Todorovska I, Dragarska K, Bogdanov J. Synthesis of Symmetrical Monocarbonyl Analogs of Curcumin Containing a 2-Bromobenzylidene Moiety and Spectrophotometric Assessment of Their Reactivity with 2-(Dimethylamino)ethanthiol. Chemistry Proceedings. 2023; 14(1):7. https://doi.org/10.3390/ecsoc-27-16084
Chicago/Turabian StyleLozanovski, Zlatko, Ivana Todorovska, Katerina Dragarska, and Jane Bogdanov. 2023. "Synthesis of Symmetrical Monocarbonyl Analogs of Curcumin Containing a 2-Bromobenzylidene Moiety and Spectrophotometric Assessment of Their Reactivity with 2-(Dimethylamino)ethanthiol" Chemistry Proceedings 14, no. 1: 7. https://doi.org/10.3390/ecsoc-27-16084
APA StyleLozanovski, Z., Todorovska, I., Dragarska, K., & Bogdanov, J. (2023). Synthesis of Symmetrical Monocarbonyl Analogs of Curcumin Containing a 2-Bromobenzylidene Moiety and Spectrophotometric Assessment of Their Reactivity with 2-(Dimethylamino)ethanthiol. Chemistry Proceedings, 14(1), 7. https://doi.org/10.3390/ecsoc-27-16084





