Conformational Study of n,n’-(Alkane-1,n-diyl)bis(2-phenyl-3,5-dihydro-4H-imidazol-4-one)s with Different Spacer Length †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Physical Measurements
2.2. Synthesis of the Bis-Imidazolones (1)
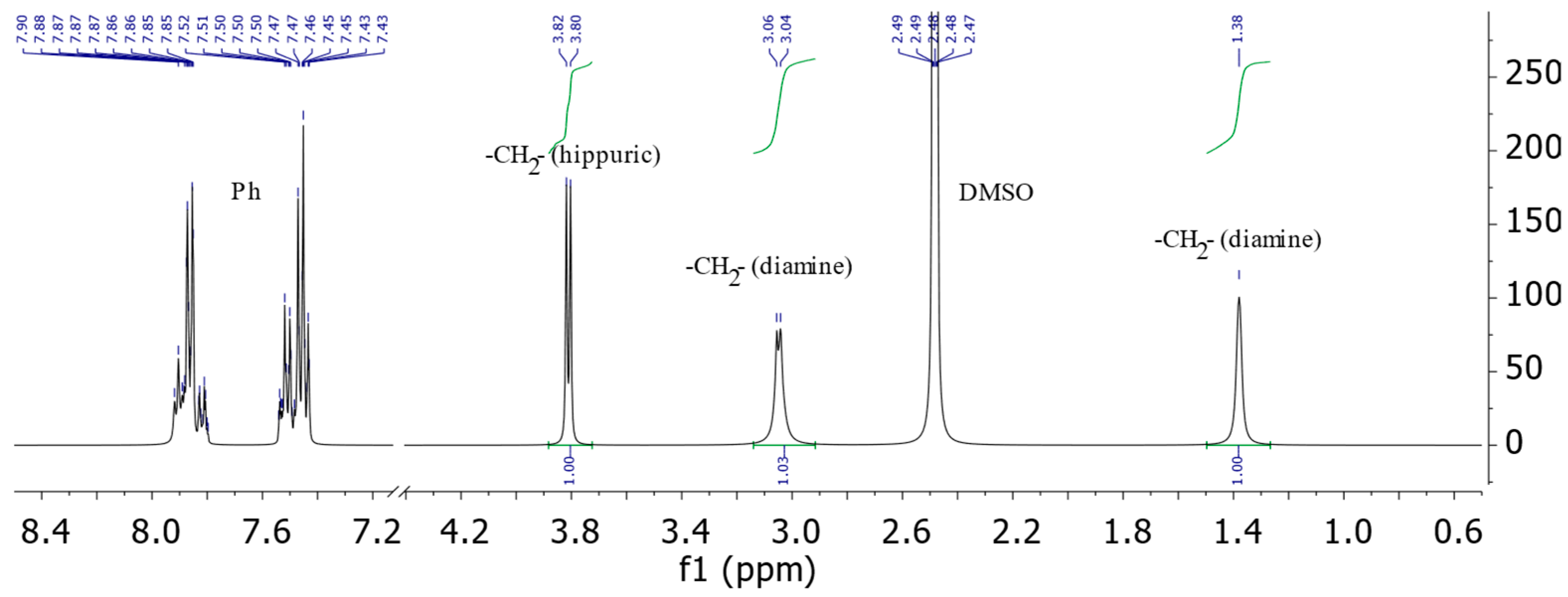
- 3,3’-(Ethan-1,2-diyl)bis(2-phenyl-3,5-dihydro-4H-imidazol-4-one) (1a): sand-colored solid; yield, 75%; m.p. 210–215 °C; 1H NMR, δ, ppm: 1.38 (s., 2H, -CH2-); 3.04–3.06 (d., J = 6 Hz, 2H, -CH2-); 3.80–3.82 (d., J = 6 Hz, 2H, -CH2-); 7.43–7.54, 7.80–7.92 (m., 5H, HAr).
- 3,3’-(Propan-1,3-diyl)bis(2-phenyl-3,5-dihydro-4H-imidazol-4-one) (1b): bright pink solid; yield 60%; m.p. 202–205 °C; 1H NMR, δ, ppm: 1.53 (s., 2H, -CH2-); 3.07–3.08 (J = 6 Hz, d., 2H, -CH2-); 3.81–3.82 (d., J = 6 Hz, 2H, -CH2-); 7.42–7.53, 7.85–7.90 (m., 5H, HAr).
- 3,3’-(Butan-1,4-diyl)bis(2-phenyl-3,5-dihydro-4H-imidazol-4-one) (1c): peach-colored solid; yield, 69%; m.p. 183–185 °C; 1H NMR, δ, ppm: 1.38 (s., 2H, -CH2-); 3.04–3.06 (J = 6 Hz, d., 2H, -CH2-); 3.80–3.82 (d., J = 6 Hz, 2H, -CH2-); 7.43–7.54, 7.80–7.92 (m., 5H, HAr).
- 3,3’-(Pentan-1,5-diyl)bis(2-phenyl-3,5-dihydro-4H-imidazol-4-one) (1d): light brown solid; yield, 64%; m.p. 172–177 °C. 1H NMR, δ, ppm: 3.05–3.06 (d., J = 6 Hz, 2H, -CH2-); 3.81–3.82 (J = 6 Hz, d., 2H, -CH2-); 7.41–7.53, 7.80–7.87 (m., 5H, HAr).
- 3,3’-(Hexan-1,6-diyl)bis(2-phenyl-3,5-dihydro-4H-imidazol-4-one) (1e): peach-colored solid; yield, 65%; m.p. 168–170 °C; 1H NMR, δ, ppm: 1.38 (s., 2H, -CH2-); 1.63–1.71 (d., J = 6 Hz, 2H, -CH2-); 3.04–3.06 (d., J = 6 Hz, 2H, -CH2-); 3.80–3.82 (d., J = 6 Hz, 2H, -CH2-); 7.43–7.54, 7.80–7.92 (m., 5H, HAr).
- 3,3’-(Heptan-1,7-diyl)bis(2-phenyl-3,5-dihydro-4H-imidazol-4-one) (1f): brick-red solid; yield, 77%; m.p. 163–165 °C; 1H NMR, δ, ppm: 3.80–3.82 (d., J = 6 Hz, 2H, -CH2-); 7.43–7.53, 7.80–7.87 (m., 5H, HAr).
2.3. DFT Calculations
2.4. Conformational Study
3. Results and Discussion
3.1. Spectral Characterization of the Synthesized bis-imidazolones (1)
3.2. DFT Calculations
3.3. Conformational Study
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Menger, F.M.; Littau, C.A. Gemini surfactants: A new class of self–assembling molecules. J. Am. Chem. Soc. 1993, 115, 10083–10090. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Frolov, N.A.; Egorova, K.S.; Seitkalieva, M.M.; Ananikov, V.P. Quaternary Ammonium Compounds (QACs) and Ionic Liquids (ILs) as Biocides: From Simple Antiseptics to Tunable Antimicrobials. Int. J. Mol. Sci. 2021, 22, 6793. [Google Scholar] [CrossRef] [PubMed]
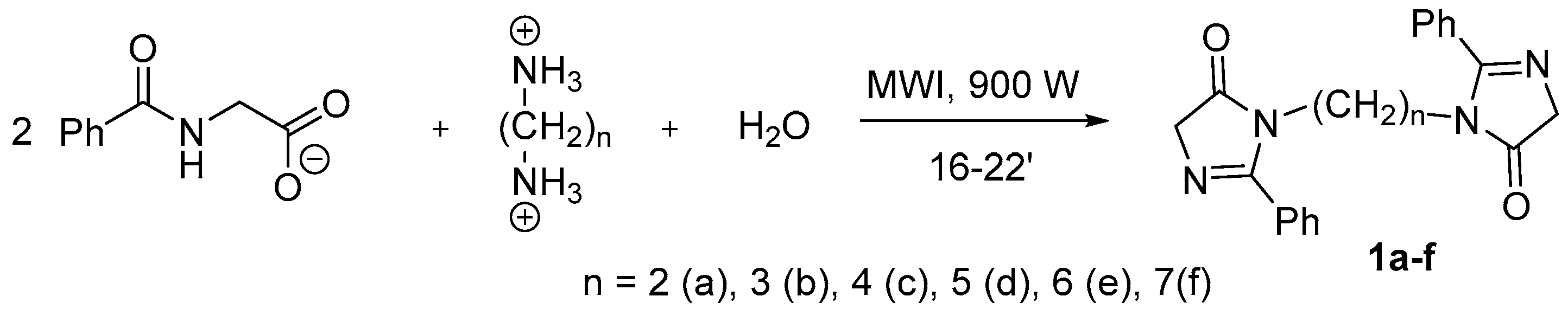
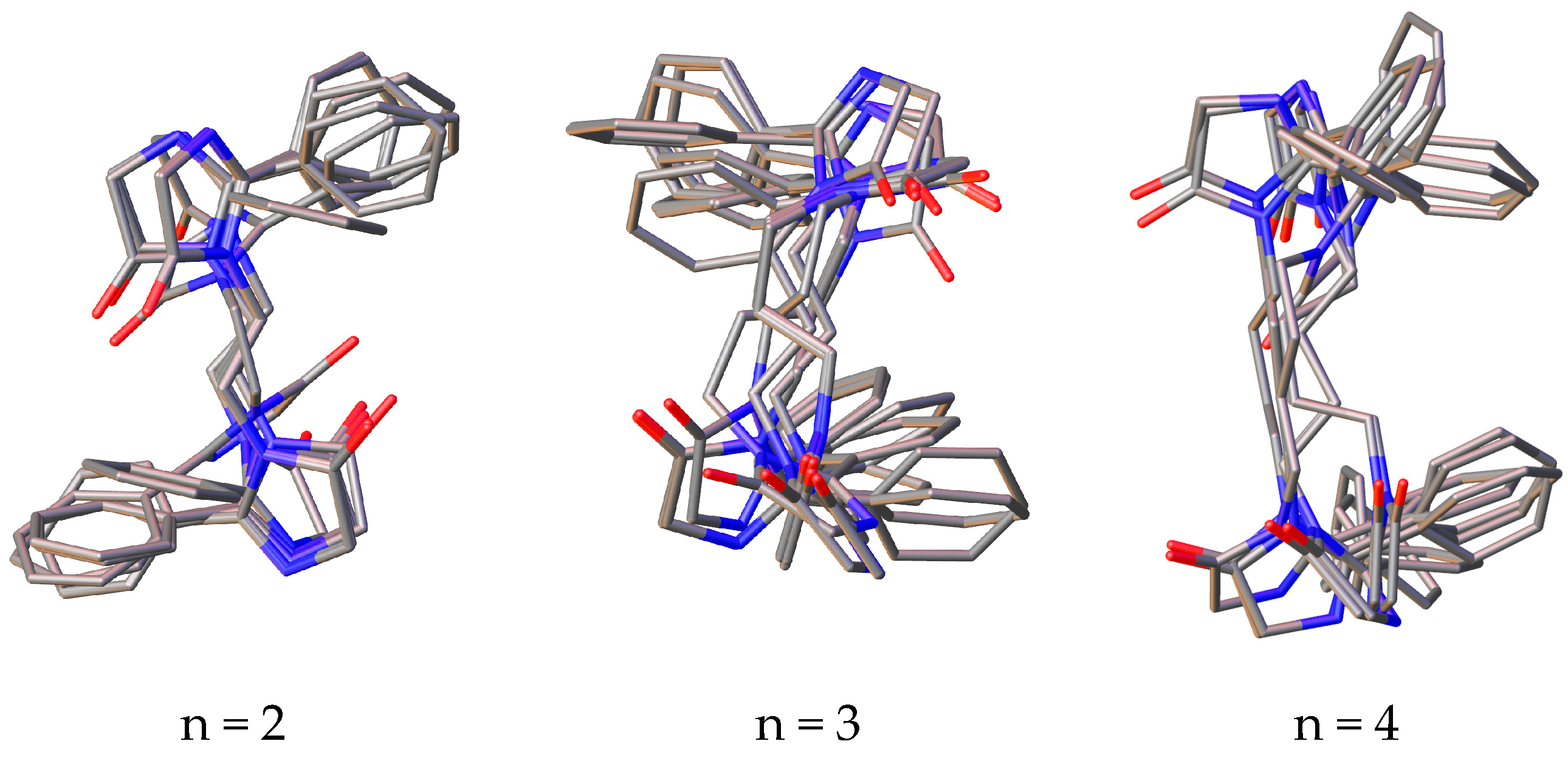
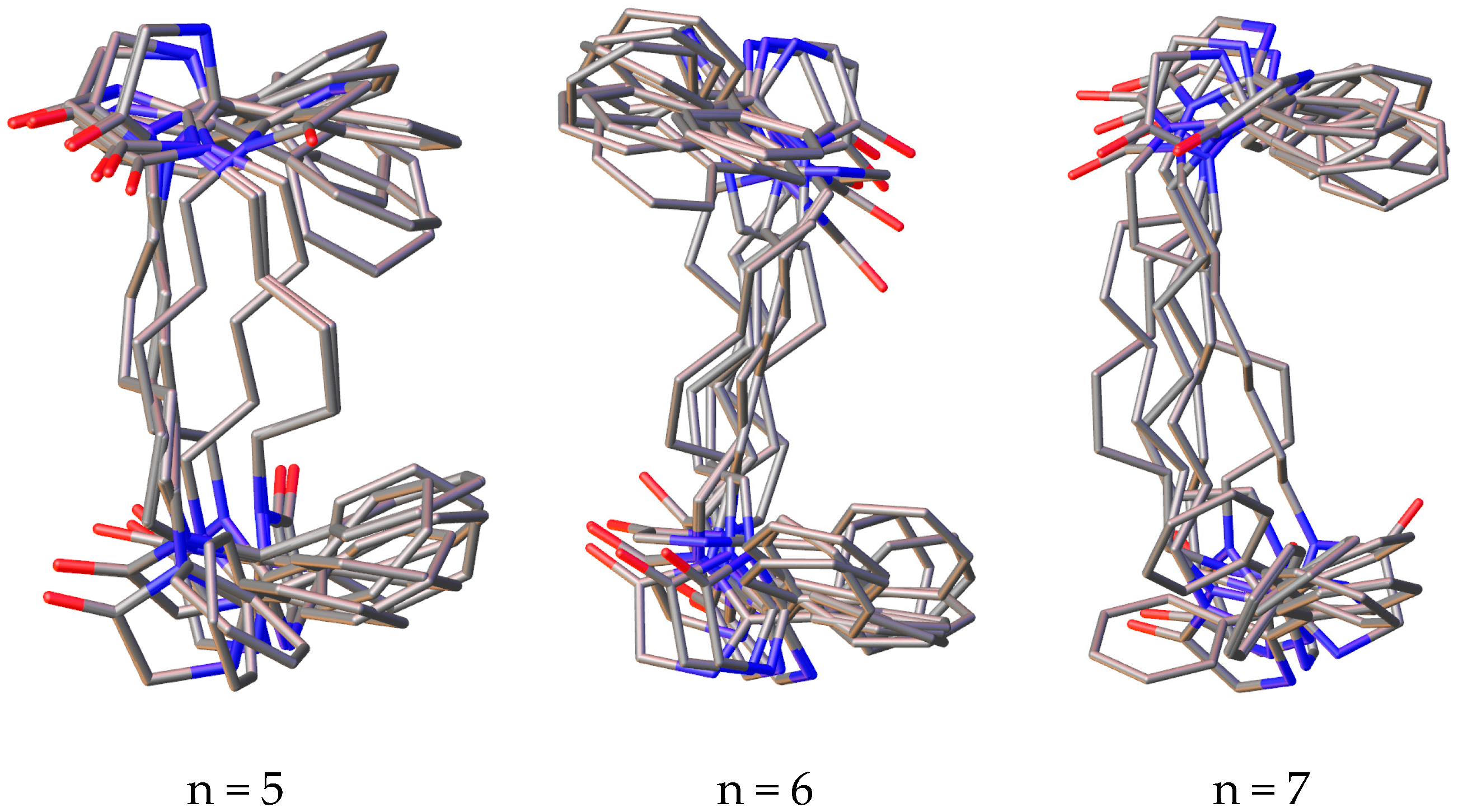
| Compd. | Distance, Å | |||
|---|---|---|---|---|
| Ph-αCH2 | Ph-βCH2 | |||
| Range | Average | Range | Average | |
| 1a | 1.452–2.249 | 2.117 | 2.602–4.560 | 4.191 |
| 1b | 1.423–2.256 | 1.985 | 1.997–4.184 | 3.184 |
| 1c | 1.337–2.324 | 2.060 | 2.124–4.439 | 3.567 |
| 1d | 1.338–2.324 | 2.011 | 2.122–4.806 | 3.489 |
| 1e | 1.322–2.322 | 2.031 | 2.201–3.722 | 3.400 |
| 1f | 1.338–2.322 | 2.091 | 2.119–4.344 | 3.887 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lobankova, A.A.; Grinev, V.S.; Yegorova, A.Y. Conformational Study of n,n’-(Alkane-1,n-diyl)bis(2-phenyl-3,5-dihydro-4H-imidazol-4-one)s with Different Spacer Length. Chem. Proc. 2023, 14, 6. https://doi.org/10.3390/ecsoc-27-16053
Lobankova AA, Grinev VS, Yegorova AY. Conformational Study of n,n’-(Alkane-1,n-diyl)bis(2-phenyl-3,5-dihydro-4H-imidazol-4-one)s with Different Spacer Length. Chemistry Proceedings. 2023; 14(1):6. https://doi.org/10.3390/ecsoc-27-16053
Chicago/Turabian StyleLobankova, Anastasia A., Vyacheslav S. Grinev, and Alevtina Yu. Yegorova. 2023. "Conformational Study of n,n’-(Alkane-1,n-diyl)bis(2-phenyl-3,5-dihydro-4H-imidazol-4-one)s with Different Spacer Length" Chemistry Proceedings 14, no. 1: 6. https://doi.org/10.3390/ecsoc-27-16053
APA StyleLobankova, A. A., Grinev, V. S., & Yegorova, A. Y. (2023). Conformational Study of n,n’-(Alkane-1,n-diyl)bis(2-phenyl-3,5-dihydro-4H-imidazol-4-one)s with Different Spacer Length. Chemistry Proceedings, 14(1), 6. https://doi.org/10.3390/ecsoc-27-16053





