Abstract
This work aimed to determine the chemical composition of hydrosol extract from Atractylis gummifera and to assess the in vitro antioxidant and anti-inflammatory properties of this extract, as well as analyze the carlina oxide isolated from it. This study also examined the in vitro synergistic effect of carlina oxide with BHT and diclofenac to reduce their minimum effective doses and minimize their potential side effects. The primary component of the hydrosol extract from A. gummifera was identified as the acetylenic compound carlina oxide (79.1%), which was isolated and confirmed through spectroscopic techniques such as 1H NMR, 13C NMR, and IR. The results of the biological assays demonstrated that both the hydrosol extract and carlina oxide exhibited noteworthy antioxidant and anti-inflammatory properties. Additionally, combining carlina oxide with positive controls yielded the enhancement of these activities, resulting in a significant reduction in the inhibitory concentrations and doses of the synthetic antioxidants and anti-inflammatories.
1. Introduction
The challenge for an ethical and environmentally conscious industrialist is to create products that are 100% natural, safe, and effective, meeting the demands of informed consumers. This involves developing eco-designed products while respecting the natural variability of the plant extracts used as ingredients. This variability impacts not only the product’s effectiveness and safety but also its stability throughout its useful lifespan.
In recent years, synthetic antioxidants have been explored in the pharmaceutical, agro-food, and cosmetics industries as potential solutions to various conditions involving oxidative stress. However, concerns have arisen about the long-term carcinogenic, teratogenic, and mutagenic effects of these synthetic antioxidants [1]. Additionally, a significant portion of anti-inflammatory drugs available on the market is chemically based. While these drugs are potent, their prolonged use can result in side effects, such as gastric intolerance [2]. Consequently, the replacement of chemical treatments with natural remedies derived from plants, such as essential oils with effects equivalent to synthetically manufactured drugs, appears promising. Essential oils are natural substances biosynthesized by aromatic plants, rich in bioactive compounds with robust biological and therapeutic effects. These effects encompass antioxidant, anticancer, anti-inflammatory, antimicrobial, antiviral, pain-relieving, and insecticidal properties [3,4,5,6,7,8]. Essential oils are also employed to prolong the shelf life of food products and serve as antioxidants, reducing susceptibility to bacterial contamination [9]. However, hydrosols, also known as the floral water byproduct obtained during essential oil hydrodistillation, have received less attention in terms of both chemical and biological research [10].
The Asteraceae family comprises a substantial variety of flowering plants, encompassing almost 1600 genera and housing over 23,000 species [11]. Among these species, Matricaria recutita L., Carlina hispanica, and Cartamus caeruleus are notably aromatic and have previously been recognized for their medicinal uses [12,13].
Within the Asteraceae family, there is a genus known as Carlina. Traditional medicine has ascribed a range of therapeutic properties to species within this genus. In numerous countries, these plants have been employed in the treatment of various skin ailments, including cancer [14]. The particular species under consideration in this study is A. gummifera L.
A. gummifera L. was recorded by Linnaeus in 1753, but the plant was already known to the ancient Greeks and Romans as the “Chameleon”. Theophrastus and Dioscorides, in fact, described in their research two types of Chameleons, the white and the black, both possessing various therapeutic and poisonous properties [15].
To explore the potential sources and applications of this plant, our study aimed to (i) isolate the hydrosol extract from A. gummifera L. and its major components, (ii) assess the in vitro anti-inflammatory activities of the hydrosol extract and its major components, and (iii) evaluate the combined effects of the major component of the essential oil with reference molecules.
2. Material and Methods
2.1. Plant Material
A. gummifera L. samples were harvested at the flowering stage in May 2022 from Oucheba Forest in Tlemcen (Algeria). The authentication of the collected plant was made by Dr. KAZI TANI Choukri, a botanist affiliated with the Department of Pharmacy at the University of Tlemcen.
2.2. Hydrosol Isolation and Liquid–Liquid Extraction (LLE)
A. gummifera L. samples (1 kg) were subjected to hydrodistillation in a Clevenger-type apparatus for 5 h. The first 500 mL of water obtained from hydrodistillation was recovered in order to obtain the corresponding hydrosol. Hydrosol was extracted three times with 200 mL of diethyl ether at room temperature. The organic layer was evaporated and dried on Na2SO4, giving a yellowish oil with yield of 0 6%.
2.3. Isolation of Components
Collective hydrosol extract (1 g) was obtained through mixing all the hydrosol extract samples, and was then subsequently subjected to column chromatography for separation, using silica gel column chromatography (FC, silica gel 200–500 μm) and eluted with 100% hexane.
2.4. Gas Chromatography
Gas chromatography (GC) analyses were performed using a Perkin-Elmer Clarus 600 GC apparatus located in Walhton, MA, USA. The instrument was equipped with a single injector and two flame ionization detectors (FID). Simultaneous sampling was conducted on two fused-silica capillary columns with the following specifications: 60 m in length, 0.22 mm in diameter, and a film thickness of 0.25 μm. These columns had different stationary phases, namely Rtx-1 (polydimethylsiloxane) and Rtx-Wax (polyethylene glycol). The temperature program for the oven was initiated at 60 °C, with a gradual increase of 2 °C per minute until 230 °C was reached. The temperature was then maintained isothermally at 230 °C for 35 min. The carrier gas used was helium, with a flow rate of 1 mL per minute. Injector and detector temperatures were held at 280 °C. Split injection was performed with a split ratio of 1:50, and the injected volume was 0.2 μL. Retention indices (RIs) of the compounds were determined using software (TotalChrom®, version 5) provided by Perkin-Elmer (Paris, France) [16].
2.5. Gas Chromatography-Mass Spectrometry Analysis (GC–MS)
The samples were subjected to analysis using a Perkin-Elmer Turbo Mass quadrupole analyzer, which was directly connected to a Perkin-Elmer Autosystem XL. This setup featured two identical fused-silica capillary columns and operated under the same gas chromatography (GC) conditions as previously detailed, with the exception of a split ratio of 1:80. Electronic impact (EI) mass spectra were generated under the following conditions: ion source temperature: 150 °C, ionization energy: 70 electronvolts (eV), mass range: 35 to 350 Daltons (Da), scan time: 1 s [17].
2.6. Component Identification and Quantification
Component identification and quantification were conducted in the following manner:
- Identification of components: component identification involved two main approaches:
(i) GC retention indices (RI) were determined by comparing them on both non-polar and polar columns. These RIs were established relative to the retention times of a series of n-alkanes using linear interpolation. The obtained RIs were then compared with those of known authentic compounds or data found in the literature [18,19]
(ii) Mass spectral data was used for computer matching with commercial mass spectral libraries [20,21], as well as a comparison with spectra from an in-house laboratory library.
- Quantification of components:
Quantification of the components within the hydrosol extract was achieved by determining peak normalization percent abundances. This process involved calculating the integrated response factors from flame ionization detection (FID) relative to a specific internal standard, tridecane (present at 0.7 g per 100 g). This allowed for accurate quantification of the components in the extract.
This methodology ensured the accurate identification and quantification of the various components within the hydrosol extract.
2.7. DPPH Free Radical Scavenging Assay
The antioxidant potential of both the hydrosol extract and carlina oxide was evaluated using the DPPH (2,2-Diphenyl Picryl-Hydrazyl) free radical scavenging assay [4]. Here is the procedure for calculating the antioxidant activity:
- Preparation of solutions:
Stock solutions of hydrosol extract and carlina oxide were prepared at a concentration of 20 g/L.
A range of different concentrations, varying between 0.5 and 20 g/L, were derived from these stock solutions.
- Reaction mixture:
A total of 1000 μL of each concentration was combined with 1000 μL of an ethanolic solution of DPPH (0.5 mM).
Incubation: The mixture was then incubated for 30 min in a dark environment at room temperature.
- Absorbance measurement:
After incubation, the absorbance of the solutions was measured at a wavelength of 517 nm using a spectrophotometer.
- Positive control:
The same concentrations were prepared for BHT (a known antioxidant) to serve as a positive control for comparison.
- Antioxidant activity calculation:
The antioxidant activity was calculated in the following way:
where AA: antioxidant activity, Abs: absorbance. The IC50s were calculated graphically by the linear regression formula of the inhibition percentages as a function of different concentrations of the sample tested.
AA (%) = [(Abscontrol − Abs test)/Abscontrol] × 100
2.8. β-Carotene Bleaching Test
The antioxidant capacity of both the hydrosol extract and carlina oxide was assessed using the β-carotene bleaching test, which measures their ability to inhibit the oxidative degradation (discoloration) of β-carotene caused by the oxidation products of linoleic acid. This test follows the method outlined by Bougatef et al. [22]. Here is a summary of the procedure and how the percentage inhibition was calculated:
- Preparation of β-Carotene/Linoleic Acid emulsion:
Initially, a β-carotene/linoleic acid emulsion was prepared by dissolving 2 mg of β-carotene in 10 mL of chloroform.
Then, 25 μL of linoleic acid and 200 mg of Tween 40 were mixed into the solution. The chloroform was completely evaporated at 40 °C using a rotavapor. Afterward, 100 mL of distilled water saturated with oxygen were added to create an emulsion, which was vigorously stirred.
- Sample preparation:
Test tubes containing 2.5 mL of the emulsion were prepared. To these test tubes, 1 mL of the extracts in ethanol at various concentrations was added.
- Heating and absorbance measurement:
The test tubes were then heated to 50 °C in a double boiler. Absorbance readings were taken for all samples at two time points: immediately (t = 0) and after 120 min, using a spectrophotometer. A blank sample, consisting of an emulsion without β-carotene, was also measured for reference.
- Percentage inhibition calculation:
The percentage inhibition was calculated using the following formula:
where AE120: absorbance at 470 nm of the samples at t = 120 min.
A% = [1 − (AE (0) − AC (120)/(AC (0) − AC (120)] × 100
- AC0: absorbance at 470 nm of the control at t = 0 min.
- AC120: absorbance at 470 nm of the control at t = 120 min.
The IC50s were calculated graphically using the linear regression formula of the inhibition percentages as a function of different concentrations of the sample tested.
2.9. Anti-Inflammatory Activity
The anti-inflammatory activity of the hydrosol extract and carlina oxide was evaluated in vitro using the protein denaturation method, with diclofenac serving as a reference anti-inflammatory drug. The procedure involved the following steps:
- Reaction mixture:
A reaction mixture was prepared, consisting of 2 mL of various dilutions of the hydrosol extract, carlina oxide, or a control solution (distilled water).
To this, 2.8 mL of phosphate-buffered saline (PBS, pH 6.4) mixed with 0.2 mL of fresh egg albumin were added.
- Incubation:
The mixture was incubated at 37 °C for 15 min.
- Denaturation:
After incubation, the denaturation of albumin was induced by placing the mixture in a water bath at 70 °C for 5 min.
- Absorbance measurement:
After cooling, the absorbance of the samples was measured at 660 nm [23,24], using a spectrophotometer.
- Percentage inhibition calculation:
The percentage inhibition of denaturation was calculated using the following formula:
% Inhibition = [(Ac − At)/Ac] × 100
- Ac: absorbance at 660 nm of control.
- At: absorbance at 660 nm of samples.
3. Results and Discussion
3.1. Chemical Composition of the Hydrosol Extract
Prepared by liquid–liquid extraction, the hydrosol extract was also analyzed by GC-IR and GC/MS, and eleven constituents representing 99.8% of the total extract composition were identified (Table 1). The hydrosol extract consists exclusively of oxygenated compounds. The primary chemical classes in the hydrosol extract are acetylenic compounds (79.1%) and non-terpenic oxygenated compounds (13.8%), followed by oxygenated diterpenes (5.8%). The major constituents are carlina oxide (79.1%), hexadecanol (10.1%), and ferruginol (5.7%). Ferruginol was first isolated in 1939 from the resin of the Miro tree (Podocarpus ferrugineus), an endemic species of New Zealand. Ferruginol (abieta-8,11,13-triene-12-ol) is the simplest phenolic abietane diterpenoid. This abietane is found in plants belonging to the families Podocarpaceae, Cupressaceae, Lamiaceae, and Verbenaceae, among others. This diterpene has garnered significant attention as it is known to possess multiple activities, including antimicrobial, miticidal, cardioactive, antioxidant, anti-leishmanial, and nematicidal properties [25].

Table 1.
Chemical composition of the hydrosol extract of A. gummifera L.
However, our spectral libraries proved ineffective in identifying the primary component of the hydrosol extract (listed as N°4 in Table 1). To address this, column chromatography, as detailed in the materials and methods section, was employed to isolate the major compound, Carlina oxide, with a mass of 0.70 g. Identification of Carlina oxide involved additional complementary analyses, including 1H NMR, 13C NMR, and IR spectra. By comparing the obtained compound with data found in the literature [27], Carlina oxide was successfully identified, also known as benzyl-2-furylacetylene (as shown in Figure 1).
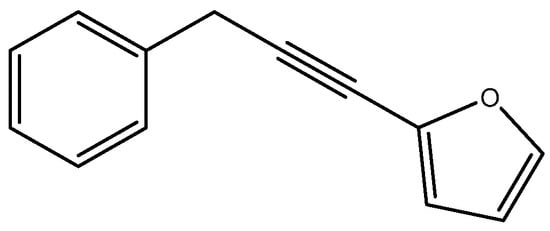
Figure 1.
Carlina oxide.
Carlina oxide was identified through several complementary analyses, including 1H NMR, 13C NMR, and IR spectra. The specific spectral data for Carlina oxide are as follows:
- IR spectrum (KBr, cm−1):
Ca=C (C≡C) stretching: 2216
C-H aromatic (Ar) stretching: 3086
C=C aromatic (Ar) bending: 1453, 1487, 1494, 1573
C=C furan (furano) stretching: 1603
C-H furan (furano) bending: 984
C-H aromatic (Ar) bending: 740
- 1H NMR (300 MHz, CDCl3, δ ppm):
3.93 (2H, singlet, CH2)
6.44 (1H, doublet-doublet, J1 = 3.24, J2 = 1.8, CH in furan)
6.62 (1H, doublet, J = 3.62, Furane C-H)
7.3–7.5 (6H, multiplet, CH in phenyl + CH in furan)
13C NMR (100 MHz, CDCl3, δ ppm):
143.05, 137.4, 135.9, 128.7, 128.1, 126.9, 114.3, 110.8, 92.1, 73.1, 25.8
3.2. Evaluation of the Antioxidant Activities
- DPPH free radical scavenging assay:
The assessment of the antioxidant capacity was carried out using the DPPH free radical scavenging method and the β-carotene bleaching method, with BHT serving as a positive control (see Figure 2).
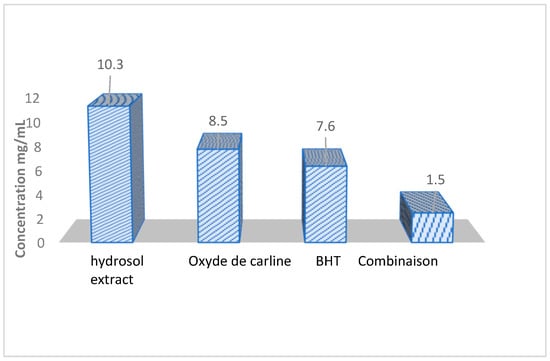
Figure 2.
IC50 Values for the hydrosol extract, Carlina Oxide, and the Carlina Oxide–BHT combination at various concentrations using the DPPH• test.
The half-maximal inhibitory concentration (IC50), which signifies the concentration providing 50% inhibition of the DPPH in the test solution, was determined. All the samples demonstrated antioxidant activity.
The combination of Carlina oxide and BHT exhibited the most robust activity in quenching the DPPH• radical, with an IC50 of 1.5 g/L. This IC50 is approximately five times lower than that of the synthetic antioxidant used as a reference, BHT (IC50 = 7.6 g/L; Figure 2). It was followed by BHT (IC50 = 7.6 g/L), Carlina oxide (IC50 = 8.5 g/L), and the hydrosol extract (IC50 = 10.3 g/L) (Figure 2).
- β-Carotene Bleaching assay
The results obtained indicate that the hydrosol extract and carlina oxide exhibit strong activity against the peroxide radicals generated during the oxidation of linoleic acid (see Figure 3). Carlina oxide displayed a more remarkable free radical scavenging activity compared to the hydrosol extract, with IC50 values of 5.2 g/L and 11.9 g/L, respectively. The synthetic antioxidant had an IC50 of approximately 4.8 g/L. Notably, the combination of carlina oxide and BHT demonstrated a synergistic effect, with an IC50 of 2.1 g/L, approximately twice as potent as BHT alone (Figure 3).
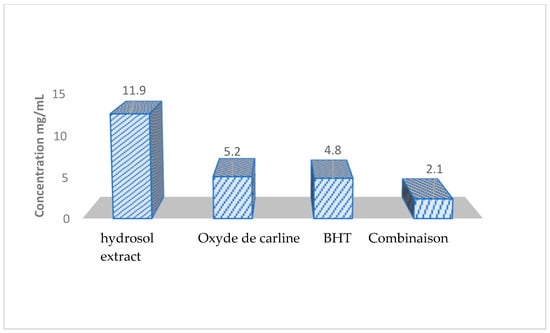
Figure 3.
Antioxidant potential of the hydrosol extract, Carlina Oxide, and the Carlina Oxide–BHT combination using the β-Carotene/Linoleic Acid bleaching assay at various concentrations.
3.3. Assessment of Anti-Inflammatory Activity
The in vitro evaluation of the anti-inflammatory activity of the hydrosol extract, carlina oxide, diclofenac sodium, and the carlina oxide–diclofenac combination was performed using the protein denaturation method (refer to Table 2). The results presented in Table 2 demonstrate a concentration-dependent inhibition of protein denaturation (albumin) by the tested samples.

Table 2.
Percentages of inhibition of protein denaturation of A. gummifera L. hydrosol extract, carlina oxide, sodium diclofenac, and their combination at different concentrations.
Diclofenac sodium was used as the reference drug at an equivalent concentration. The findings reveal that the hydrosol extract and carlina oxide exhibit notable inhibitory effects, with percentages of 78.2% and 80.5%, respectively, at a concentration of 15 g/L, in comparison to Diclofenac (65.8%) at the same concentration. Notably, the combination of carlina oxide and Diclofenac displayed a strong inhibitory effect (90.1%) at the same concentration of 15 g/L (Table 2).
4. Conclusions
The uniqueness of this work lies in the fact that, for the first time, the chemical composition and biological properties of hydrosol extract extracted from Atractylis gummifera has been described. The chemical analysis revealed that hydrosol extract is notably abundant in acetylenic compounds. The in vitro experiments combining carlina oxide with BHT exhibited improved efficacy, resulting in a significant reduction in the inhibitory concentration. Both the hydrosol extract and carlina oxide displayed promising anti-inflammatory activity, with the combination of carlina oxide and diclofenac showing superior results. The findings suggest that combining carlina oxide with positive controls can lower the minimum effective dosage, potentially reducing side effects while maintaining comparable potency. Therefore, further research should involve additional assessments of the therapeutic effectiveness of the hydrosol extract, carlina oxide, and their combinations, utilizing in vivo tests.
Author Contributions
All authors contributed equally to this work. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The dataset used and/or analyzed during the current study are available from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lewis, K. Antibiotics: Recover the lost art of drug discovery. Nature 2012, 485, 439–440. [Google Scholar] [CrossRef]
- Ndoye Foe, F.M.C.; Kemegni Tchinang, T.F.; Nyegue, A.M.; Abdou, J.-P.; Gbaweng Yaya, A.J.; Tchinda, A.T.; Oyono Essame, J.-L.; Etoa, F.-X. Chemical composition, in vitro antioxidant and anti-inflammatory properties of essential oils of four dietary and medicinal plants from Cameroon. BMC Complement. Altern. Med. 2016, 16, 117. [Google Scholar] [CrossRef]
- Bardeau, F. Les Huiles Essentielles: Découvrir les Bienfaits et les Vertus d’une Médecine Ancestrale; Fernand Lanore: Nancy, France, 2009. [Google Scholar]
- El-Massry, K.F.; Farouk, A.; Abou-Zeid, M. Free radical scavenging activity and lipoxygenase inhibition of rosemary (Rosmarinus officinalis L.) volatile oil. J. Essent. Oil Bear. Plant. 2008, 11, 536–543. [Google Scholar] [CrossRef]
- Medjdoub, K.; Benomari, F.Z.; Djabou, N.; Dib, M.A.; Gaouar Benyelles, N.; Costa, J.; Muselli, A. Antifungal and insecticidal activities of essential oils of four Mentha species. Jundishapur J. Nat. Pharm. Prod. 2019, 14, e64165. [Google Scholar] [CrossRef]
- Tefiani, C.; Riazi, A.; Youcefi, F.; Aazza, S.; Gago, C.; Faleiro, M.L.; Pedro, L.G.; Barroso, J.G.; Figueiredo, A.C.; Megías, C.; et al. Ammoides pusilla (Apiaceae) and Thymus munbyanus (Lamiaceae) from Algeria essential oils: Chemical composition, antimicrobial, antioxidant and antiproliferative activities. J. Essent. Oil Res. 2015, 27, 1–9. [Google Scholar] [CrossRef]
- Willem, J.P. Huiles Essentielles Antivirales; Guy Trédaniel: Paris, France, 2015. [Google Scholar]
- Mouhi, L.; Moghrani, H.; Nasrallah, N.; Amrane, A.; Maachi, R. Anti inflammatory activity of essential oil of an endemic Thymus fontanesii Boiss. & Reut. with chemotype carvacrol, and its healing capacity on gastric lesions. J. Food Biochem. 2017, 41, e12359. [Google Scholar]
- Merad Boussalah, N. Chemical Composition and Biological Activities of Essential Oil and Hydrosol Extract from Aerial Parts of Cynoglossum cheirifolium L. from Algeria. J. Essent. Oil Bear. Plant. 2020, 23, 97–104. [Google Scholar] [CrossRef]
- Ainseba, N.; Tabet Zatla, A.; Dib, M.E.A.; Tabti, B.; Costa, J.; Muselli, A. Antifungal Activity of Essential Oil and Hydrosol Extract of Ballota nigra L. and their Protective Effects Against the Black Rot of Tomatoes. Curr. Nutr. Food Sci. 2018, 14, 1–10. [Google Scholar]
- Barreda, L.P.; Maria, C.T.; Eduardo, B.O.; Ian, R.; Félix, F.; Viviana, D. Early evolution of the angiosperm clade Asteraceae in the Cretaceous of Antarctica. Proc. Natl. Acad. Sci. USA 2015, 112, 10989–10994. [Google Scholar] [CrossRef] [PubMed]
- Achiri, R.; Benhamidat, L.; Mami, I.R.; Dib, M.E.A.; Aissaoui, N.; Ziani Cherif, C.; Ziani Cherif, H.; Muselli, A. Chemical Composition and Antioxidant, Anti-inflammatory and Antimicrobial Activities of the Essential Oil and its Major Component (Carlina oxide) of Carlina hispanica Roots from Western Algeria. J. Essent. Oil Bear. Plants 2021, 24, 1113–1124. [Google Scholar] [CrossRef]
- Kenny, O.; Smyth, T.J.; Walsh, D.; Kelleher, C.T.; Hewage, C.M.; Brunton, N.P. Investigating the potential of under-utilised plants from the Asteraceae family as a source of natural antimicrobial and antioxidant extracts. Food Chem. 2014, 161, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Strzemski, M.; Wojnicki, K.; Sowa, I.; Wojas-Krawczyk, K.; Pawe Krawczyk, P.; Kocjan, R.; Justyna Such, J.; Latalski, M.; Wnorowski, A.; Wójciak-Kosio, M. In Vitro Antiproliferative Activity of Extracts of Carlina acaulis subsp. caulescens and Carlina acanthifolia subsp. utzka. Front. Pharmacoly 2017, 8, 371. [Google Scholar]
- Daniele, C.; Dahamna, S.; Firuzi, O.; Sekfali, N.; Saso, L.; Mazzanti, G. Atractylis gummifera L. poisoning: An ethnopharmacological. J. Ethnopharmacol. 2005, 97, 17581. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Standards and Technology. NIST Chemistry WebBook; NIST: Gaisthersburg, MD, USA, 2005; Standard Reference Database. Available online: http://webbook.nist.gov/chemistry (accessed on 6 January 2017).
- National Institute of Standards and Technology. NIST/EPA/NIH Mass Spectra Library, PC Version 1.7; Perkin-Elmer Corp: Norwalk, CT, USA, 1999. [Google Scholar]
- Jennings, W.; Shibamoto, T. Qualitative Analysis of Flavour and Fragrance Volatiles by Glass-Capillary Gas Chromatography, 1st ed.; Jovanovich, H.B., Ed.; Academic Press: New York, NY, USA, 1980. [Google Scholar]
- König, W.A.; Hochmuth, D.H.; Joulain, D. Terpenoids and Related Constituents of Essential Oils, 1st ed.; Library of Mass Finder 2.1; Institute of Organic Chemistry: Hamburg, Germany, 2001. [Google Scholar]
- Mc Lafferty, F.W.; Stauffer, D.B. The Wiley/NBS Registry of Mass Spectra Data, 1st ed.; Wiley-Interscience: New York, NY, USA, 1988. [Google Scholar]
- Mc Lafferty, F.W.; Stauffer, D.B. Wiley Registry of Mass Spectral Data, 6th ed.; Mass Spectrometry Library Search System Bench-Top/PBM version 3.10d; Palisade: Newfield, NY, USA, 1994. [Google Scholar]
- Bougatef, A.; Hajji, M.; Balti, R.; Lassoued, I.; Triki-Ellouz, Y.; Nasri, M. Antioxidant and free radical scavenging activities of smooth hound (Mustelus mustelus) muscle protein hydrolysates obtained by gastrointestinal proteases. Food Chem. 2009, 114, 1198–1205. [Google Scholar] [CrossRef]
- Chandra, S.; Chatterjee, P.; Dey, P.; Bhattacharya, S. Evaluation of anti-inflammatory effect of ashwagandha: A preliminary study in vitro. Pharmacogn. J. 2012, 4, 47–49. [Google Scholar] [CrossRef]
- Mizushima, Y.; Kobayashi, M. Interaction of anti-inflammatory drugs with serum proteins, especially with some biologically active proteins. J. Pharm. Pharmacol. 1968, 20, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A. Aromatic Abietane Diterpenoids: Their Biological Activity and Synthesis; The Royal Society of Chemistry: London, UK, 2015. [Google Scholar]
- Joulain, D.; König, W. The Atlas of Spectral Data of Sesquiterpene Hydrocarbons; E.B. Verlag: Hambourg, Germany, 1998. [Google Scholar]
- Djordjevic, S.; Petrovic, S.; Ristic, M.; Djokovic, D. Composition of Carlina acanthifolia Root Essential Oil. Chem. Nat. Comp. 2005, 44, 410–412. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).