New Azo Carboxylic Dyes Derived from Eugenol: Synthesis and Preliminary Application to Polyamide †
Abstract
:1. Introduction
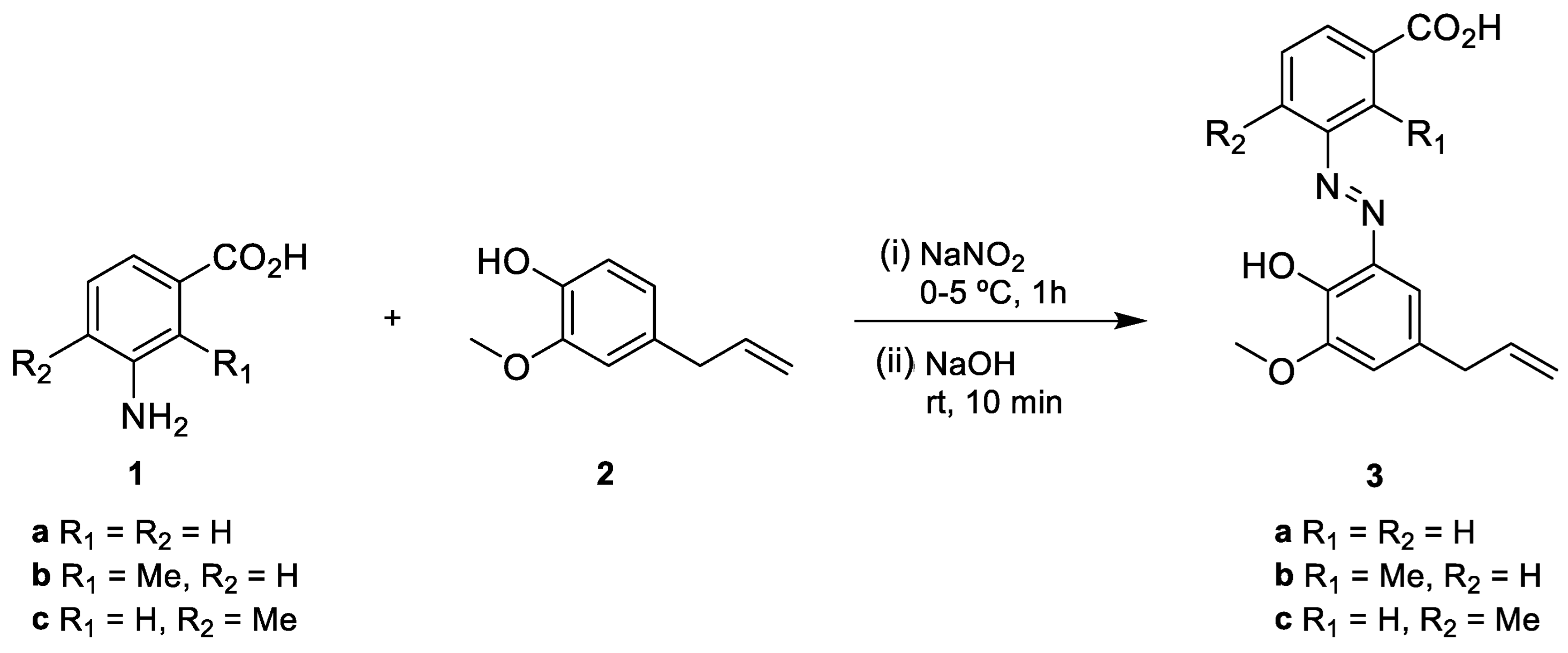
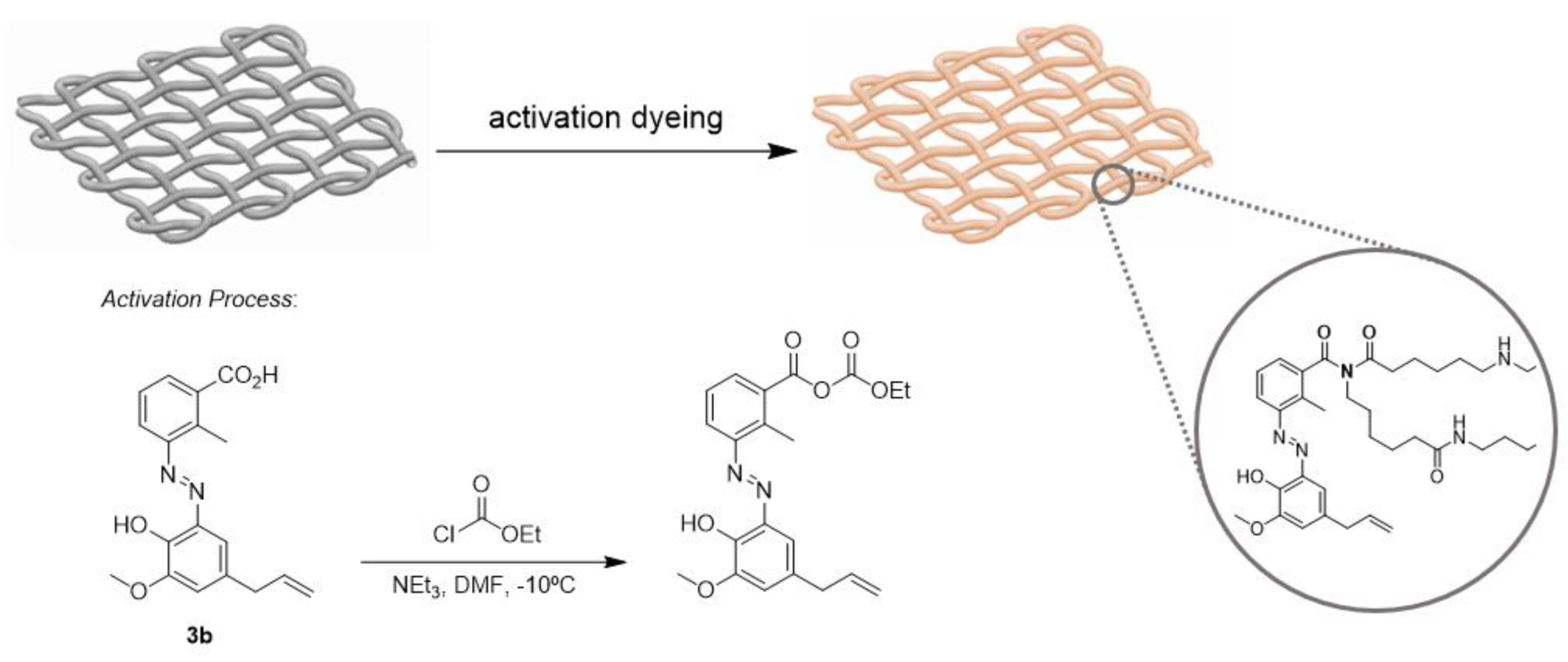
2. Results and Discussion
Synthesis of Eugenol Derivatives
3. Experimental Section
3.1. Typical Procedure for the Preparation of Compounds 3a–c (Illustrated for 3a)
3.2. Typical Procedure for Dyeing of Polyamide Fabric (Illustrated for 3a)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pizzicato, B.; Pacifico, S.; Cayuela, D.; Mijas, G.; Riba-Moliner, M. Advancements in sustainable natural dyes for textile applications: A review. Molecules 2023, 28, 5954. [Google Scholar] [CrossRef] [PubMed]
- Mouro, C.; Gomes, A.P.; Costa, R.V.; Moghtader, F.; Gouveia, I.C. The sustainable bioactive dyeing of textiles: A novel strategy using bacterial pigments, natural antibacterial ingredients, and deep eutectic solvents. Gels 2023, 9, 800. [Google Scholar] [CrossRef] [PubMed]
- Vespignani, L.; Bonanni, M.; Marradi, M.; Pizzo, B.; Bianchini, R.; Goli, G. Naturalized dyes: A new opportunity for the wood colouring. Polym. J. 2023, 15, 3632. [Google Scholar] [CrossRef] [PubMed]
- Ryzhkov, A.I.; Fedor, V.; Drozdov, F.V.; Georgij, V.; Cherkaev, G.V.; Buzin, M.I.; Svidchenko, E.A.; Muzafarov, A.M. Synthesis and properties of new siloxane with terminal azo dyes functions based on eugenol. J. Appl. Polym. Sci. 2022, 139, e52340. [Google Scholar] [CrossRef]
- Drozdov, F.V.; Cherkaev, G.V.; Mihail, I.; Buzin, M.I.; Muzafarov, A.M. Facile methods of siloxanes derivatives modification by azo dyes based on eugenol. J. Organomet. Chem. 2018, 871, 135–139. [Google Scholar] [CrossRef]
- Kantar, C.; Baltas, N.; Karaoglu, S.A.; Sasmaz, S. Some azo dyes containing eugenol and guaiacol, synthesis, antioxidant capacity, urease inhibitory properties and anti-helicobacter pylori activity. Rev. Roum. Chim. 2018, 63, 189–197. [Google Scholar]
- Benkhaya, S.; M’rabet, S.; El Harfi, A. Classifications, properties, recent synthesis and applications of azo dyes. Heliyon 2020, 6, e03271. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Sheikh, J. Synthesis of a novel cationic dye to impart mosquito-repellent and UV protection to an acrylic fabric. ACS Omega 2023, 8, 10214–10224. [Google Scholar] [CrossRef] [PubMed]
- Tahir, T.; Ashfaq, M.; Saleem, M.; Rafiq, M.; Shahzad, M.I.; Kotwica-Mojzych, K.; Mojzych, M. Pyridine scaffolds, phenols and derivatives of azo moiety: Current therapeutic perspectives. Molecules 2021, 26, 4872. [Google Scholar] [CrossRef] [PubMed]
- Mezgebe, K.; Mulugeta, E. Synthesis and pharmacological activities of azo dye derivatives incorporating heterocyclic scaffolds: A review. RSC Adv. 2022, 12, 25932–25946. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, M.S.T.; Oliveira-Campos, A.M.F.; Moura, J.C.V.P.; Maia, H.L.S.; Gomes, J.I.N.R.; Hrdina, R. Reaction of carboxylic dyes with wool and polyamide. Part III: Effect of the activating agent. Dyes Pigm. 1998, 36, 373–379. [Google Scholar] [CrossRef]
- Norma Portuguesa NP EN ISO 105-C06:1999; Têxteis: Ensaios de Solidez Dos Tintos: Parte C06: Solidez Dos Tintos à Lavagem Doméstica e Industrial: (ISO 105-C06:1999). Instituto Português da Qualidade: Almada, Portugal, 1999.
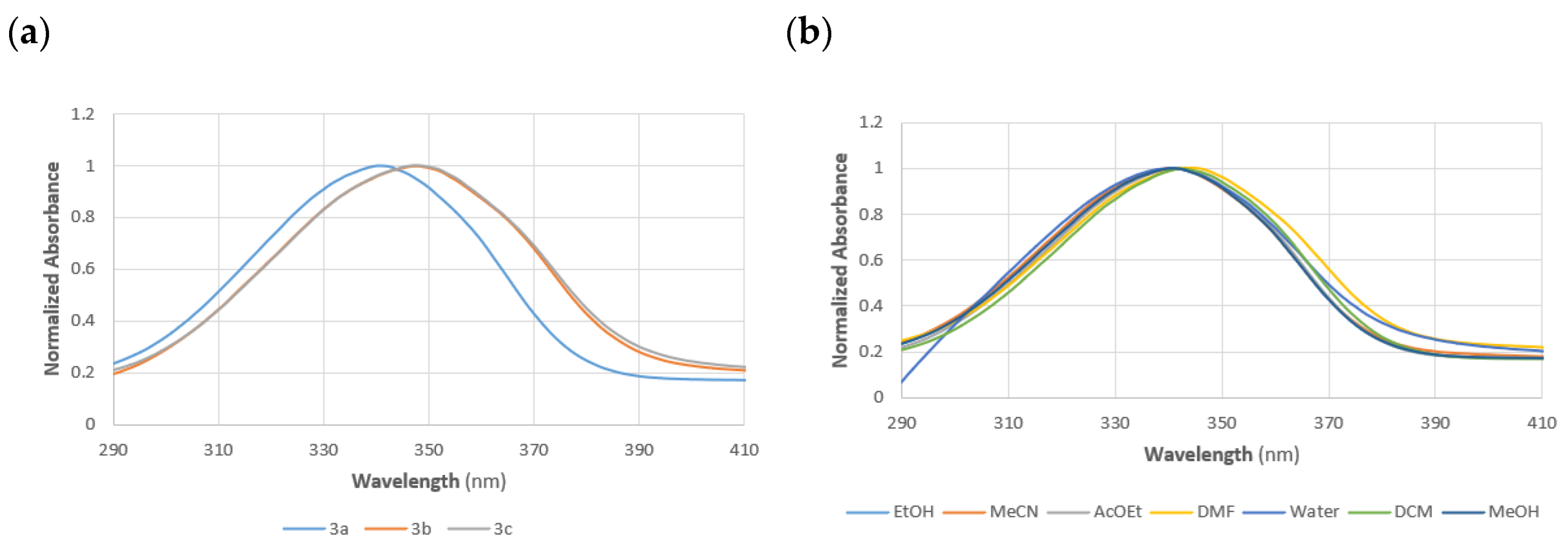
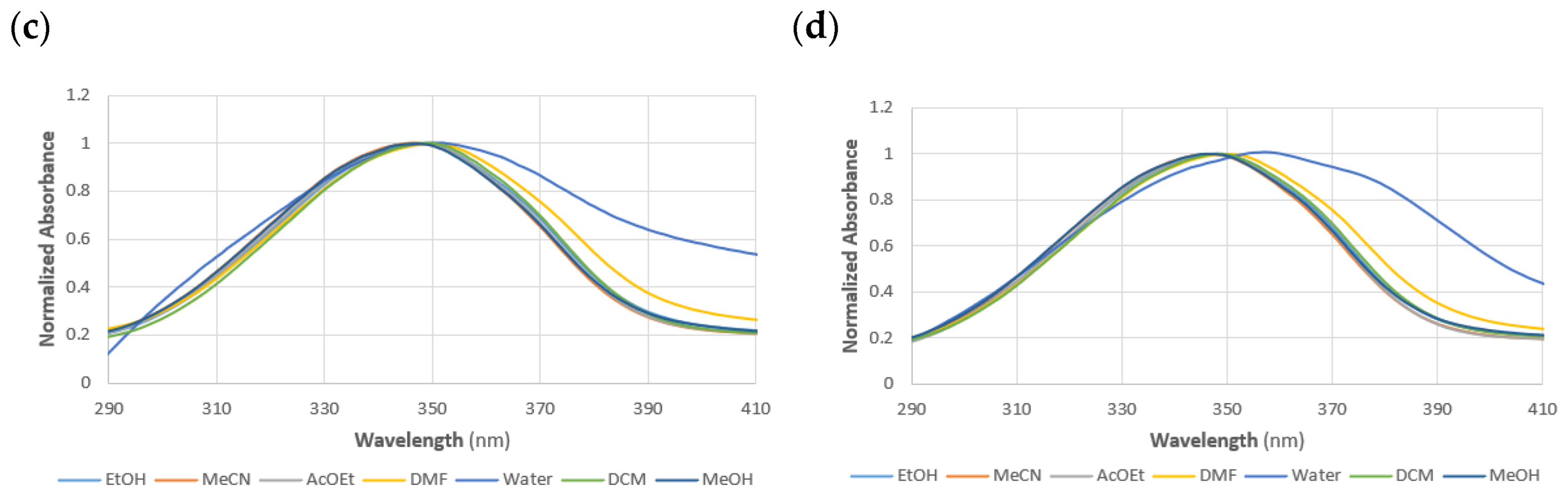
| Solvent | Dye | |||||
|---|---|---|---|---|---|---|
| 3a | 3b | 3c | ||||
| λmax | log ε | λmax | log ε | λmax | log ε | |
| EtOH | 342 | 4.34 | 347 | 4.26 | 347 | 4.18 |
| MeCN | 341 | 4.23 | 347 | 4.27 | 347 | 4.25 |
| AcOEt | 342 | 4.25 | 347 | 4.28 | 348 | 4.19 |
| DMF | 344 | 4.16 | 350 | 4.22 | 350 | 4.18 |
| H2O | 341 | 3.84 | 350 | 3.88 | 357 | 4.10 |
| DCM | 342 | 4.39 | 349 | 4.21 | 348 | 4.20 |
| MeOH | 341 | 4.34 | 347 | 4.23 | 346 | 4.17 |
| Dye | Change in Shade | Staining | |
|---|---|---|---|
| Wool | Cotton | ||
| 3a | 3 | 5 | 5 |
| 3b | 4 | 5 | 5 |
| 3c | 3 | 5 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho, J.R.A.; Fernandes, M.J.G.; Gonçalves, M.S.T. New Azo Carboxylic Dyes Derived from Eugenol: Synthesis and Preliminary Application to Polyamide. Chem. Proc. 2023, 14, 56. https://doi.org/10.3390/ecsoc-27-16044
Coelho JRA, Fernandes MJG, Gonçalves MST. New Azo Carboxylic Dyes Derived from Eugenol: Synthesis and Preliminary Application to Polyamide. Chemistry Proceedings. 2023; 14(1):56. https://doi.org/10.3390/ecsoc-27-16044
Chicago/Turabian StyleCoelho, José R. A., Maria José G. Fernandes, and M. Sameiro T. Gonçalves. 2023. "New Azo Carboxylic Dyes Derived from Eugenol: Synthesis and Preliminary Application to Polyamide" Chemistry Proceedings 14, no. 1: 56. https://doi.org/10.3390/ecsoc-27-16044
APA StyleCoelho, J. R. A., Fernandes, M. J. G., & Gonçalves, M. S. T. (2023). New Azo Carboxylic Dyes Derived from Eugenol: Synthesis and Preliminary Application to Polyamide. Chemistry Proceedings, 14(1), 56. https://doi.org/10.3390/ecsoc-27-16044







