Microwave-Assisted Synthesis, Characterization, and Biological Activity of New Copper (II) Complex with Sulfonamide †
Abstract
:1. Introduction
2. Materials and Methods
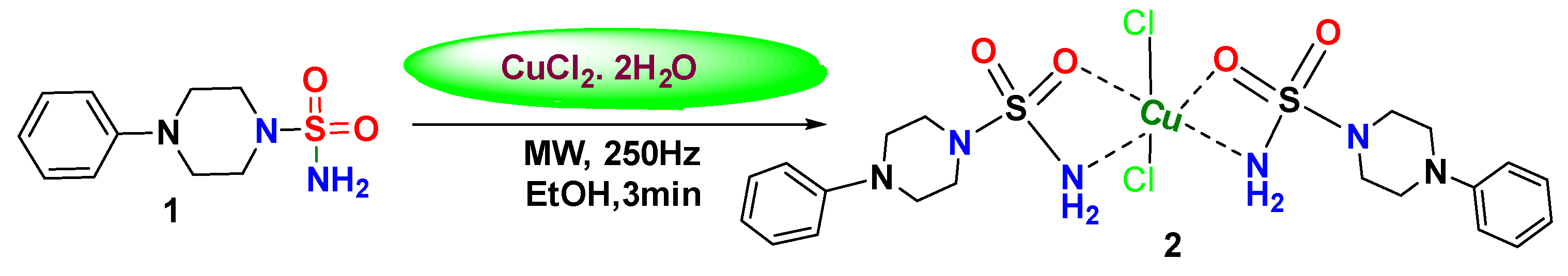
2.1. General Procedure for the Synthesis of the Complex
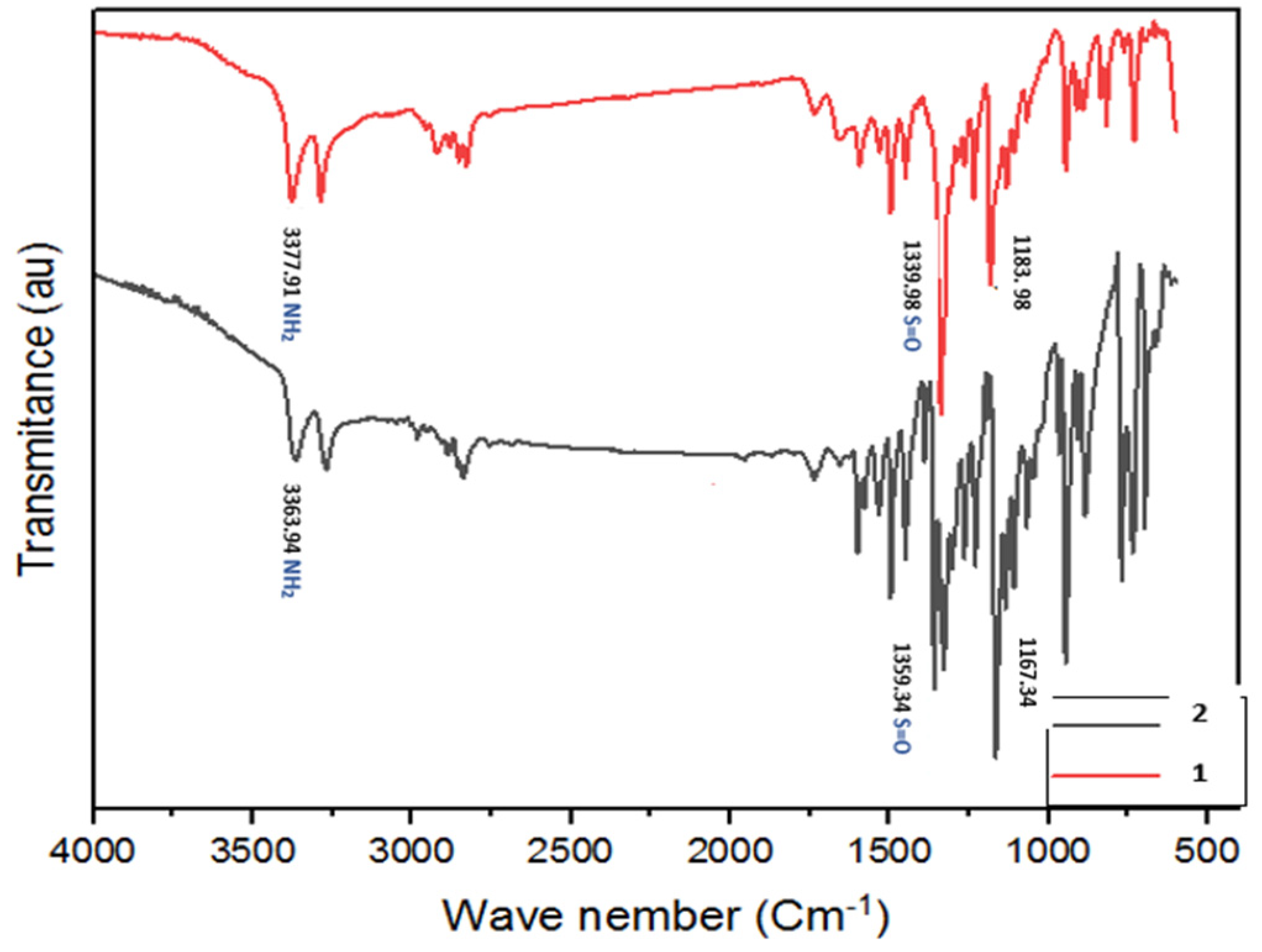
- IR (KBr): ν = 3377.91 (NH2); 1339.98 and 1183.98 (SO2) cm−1.
- Anal. Calc. for [Cu(C10H15N3O2S)2;2Cl]: C, 38.93; H, 4.90; N, 13.62; Cu, 10.30; Cl, 11.49; Found: C, 40.98; H, 5.54; N, 14.40; Cu, 11.63; Cl, 12.03.
2.2. Anti-Inflammatory Activity
- -
- Sample: 0.5 mL extract + 0.5 mL BSA.
- -
- White: 0.5 mL extract + 0.5 mL Tris-phosphate (pH: 6.8).
- -
- Control: 0.5 H2O + 0.5 mL BSA. The control represents 100% of the denatured proteins, and the results are compared to 75 mg of voltarene.
2.3. Antimicrobial Activity Test
3. Results and Discussion
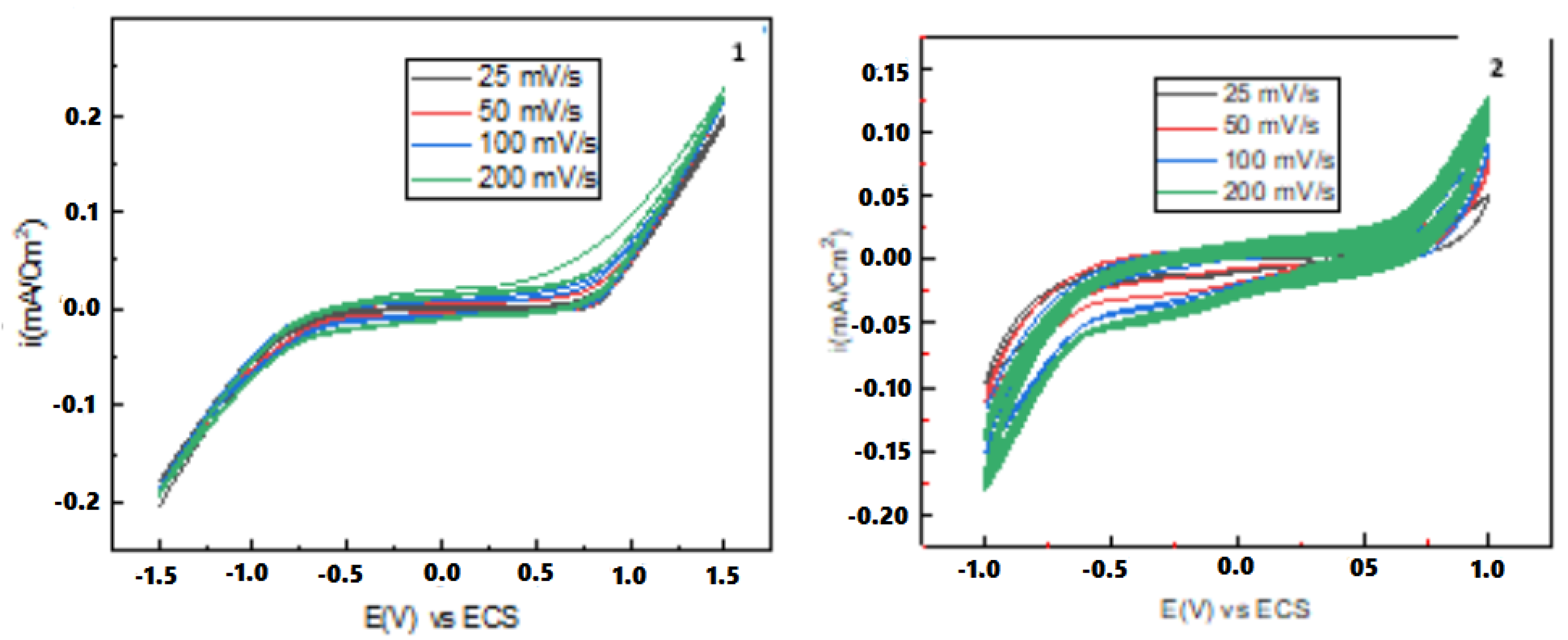
3.1. Synthesis
3.2. Anti-Inflammatory Activity
3.3. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berredjem, M.; Bouchareb, F.; Djouad, S.E.; Bouasla, R.; Bahadi, R.; Redjemia, R.; Bousaker, M.; Dekir, A. Recent Progress in Synthesis of Sulfonamides and N-Acylsulfonamides, Biological Applications and Their Structure-Activity Relationship (SAR) Studies. Chemistryselect 2023, 8, e202301859. [Google Scholar] [CrossRef]
- Gadad, A.K.; Mahajanshetti, C.S.; Nimbalkar, S.; Raichurkar, A. Synthesis and antibacterial activity of some 5-guanylhydrazone/thiocyanato-6-arylimidazo [2,1-b]-1,3, 4-thiadiazole-2-sulfonamide derivatives. Eur. J. Med. Chem. 2000, 35, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, V.K.; Srivastava, R.; Khadikar, P.V. QSAR studies on some antimalarial sulfonamides. Bioorg. Med. Chem. 2001, 9, 3287–3293. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, M.; Khadikar, P.V.; Scozzafava, A.; Supuran, C.T. Carbonic anhydrase inhibitors: The first QSAR study on inhibition of tumor-associated isoenzyme IX with aromatic and heterocyclic sulfonamides. Bioorg. Med. Chem. Lett. 2004, 14, 3283–3290. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.; Srikanth, K.; Banerjee, S.; Debnath, B.; Gayen, S.; Jha, T. 5-N-Substituted-2-(substituted benzenesulphonyl) glutamines as antitumor agents. Part II: Synthesis, biological activity and QSAR study. Bioorg. Med. Chem. 2004, 6, 1413–1423. [Google Scholar] [CrossRef] [PubMed]
- Sondhi, S.M.; Dinodia, M.; Jain, S.; Kumar, A. Synthesis of biologically active novel bis Schiff bases, bis hydrazone and bis guanidine derivatives. Indian J. Chem. 2010, 48, 1128–1136. [Google Scholar]
- Ignat, A.; Zaharia, V.; Mogosan, C.; Palibroda, N.; Cristea, C.; Silaghi-Dumitrescu, L. Heterocycles 25. Microwave assisted synthesis of some p-toluensulfonylhydrazinothiazoles with analgesic and anti-inflammatory activity. Farmacia 2010, 58, 290–302. [Google Scholar]
- Abdo, M.R.; Vullo, D.; Saada, M.C.; Montero, J.L.; Scozzafava, A.; Winum, J.Y.; Supuran, C.T. Carbonic Anhydrase Activators: Activation of Human Isozymes I, II and IX with Phenylsulfonylhydrazido l-Histidine Derivatives. Bioorg. Med. Chem. Lett. 2009, 19, 2440–2443. [Google Scholar] [CrossRef] [PubMed]
- Kremer, E.; Facchin, G.; Estévez, E.; Alborés, P.; Baran, E.J.; Ellena, J.; Torre, M.H. Synthesis, spectroscopic characterization, microbiological and SOD-like activities: Crystal structure of [Cu(sulfisoxazole)2(H2O)4]·2H2O. J. Inorg. Biochem. 2006, 100, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, A.M. Structural and biological aspects of copper (II) complexes with 2-methyl-3-amino-(3 H)-quinazolin-4-one. J. Inorg. Biochem. 1997, 65, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Chohan, Z.H.; Pervez, H.; Rauf, A.; Khalid, K.M.; Supuran, C.T. Isatin-derived antibacterial and antifungal compounds and their transition metal complexes. J. Enzyme Inhib. Med. Chem. 2004, 19, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.; Nagashri, K.; Boomadevi Janaki, G. Novel metal based anti-tuberculosis agent: Synthesis, characterization, catalytic and pharmacological activities of copper complexes. Eur. J. Med. Chem. 2012, 49, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.; Xiong, R.; Chen, Z.; Zhang, P.; Ju, H.; Dai, Z.; Guo, Z.; Fun, H.; You, X. Crystal structure of zinc(II) 2-sulfanilamidopyrimidine: A widely used topical burn drug. J. Chem. Soc. Dalton Trans. 2001, 6, 774. [Google Scholar] [CrossRef]
- Lekouaghet, A.; Boutefnouchet, A.; Bensuici, C.; Gali, L.; Ghenaiet, K.; Tichati, L. In vitro evaluation of antioxidant and anti-inflammatory activities of the hydroalcoholic extract and its fractions from Leuzea conifera L. roots. S. Afr. J. Bot. 2020, 132, 103–107. [Google Scholar] [CrossRef]
- Benzaid, C.; Tichati, L.; Djeribi, R.; Rouabhia, M. Evaluation of the Chemical Composition, the Antioxidant and Antimicrobial Activities of Mentha × piperita Essential Oil against Microbial Growth and Biofilm Formation. J. Essent. Oil Bear. Plants 2019, 22, 335–346. [Google Scholar] [CrossRef]
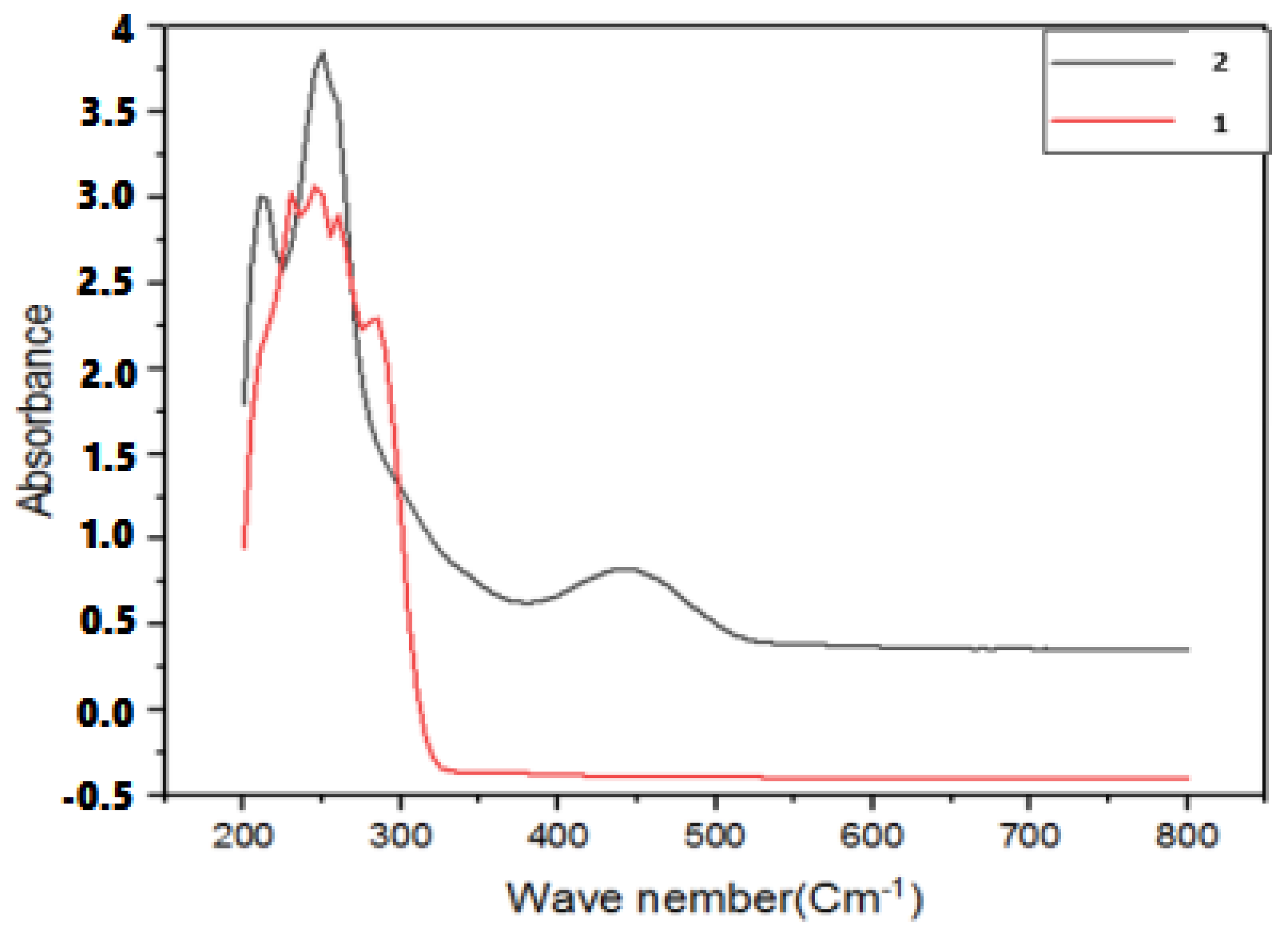
| Compound | λ (nm) | Electronic Transitions |
|---|---|---|
| Ligand | 230 | π → π* |
| 300 | π → π* | |
| Complex | 220–260 | π → π* |
| 400–470 | charge transfer |
| PPM | %Inhibition | |
|---|---|---|
| Molecule | Voltarene (75 mg) | |
| 5000 | 67.32 | 100 |
| 1500 | 31.09 | 90 |
| 1250 | 18.98 | 58 |
| 625 | 5.76 | 30 |
| Compounds | S. aureus | Streptococcus sp. | A. baumannii | K. pneumonaie | E. coli | P. aeruginosa | C. albicans | Candida sp. |
|---|---|---|---|---|---|---|---|---|
| BRC | 12 | 8 | R | 24 | R | R | 33 | 41 |
| Ampicillin | R | R | 128 | 64 | R | 32 | 32 | 32 |
| Amphoterin b | R | R | R | R | R | R | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahadi, R.; Berredjem, M.; Redjemia, R.; Benzaid, C. Microwave-Assisted Synthesis, Characterization, and Biological Activity of New Copper (II) Complex with Sulfonamide. Chem. Proc. 2023, 14, 62. https://doi.org/10.3390/ecsoc-27-16157
Bahadi R, Berredjem M, Redjemia R, Benzaid C. Microwave-Assisted Synthesis, Characterization, and Biological Activity of New Copper (II) Complex with Sulfonamide. Chemistry Proceedings. 2023; 14(1):62. https://doi.org/10.3390/ecsoc-27-16157
Chicago/Turabian StyleBahadi, Rania, Malika Berredjem, Rayenne Redjemia, and Chahrazed Benzaid. 2023. "Microwave-Assisted Synthesis, Characterization, and Biological Activity of New Copper (II) Complex with Sulfonamide" Chemistry Proceedings 14, no. 1: 62. https://doi.org/10.3390/ecsoc-27-16157
APA StyleBahadi, R., Berredjem, M., Redjemia, R., & Benzaid, C. (2023). Microwave-Assisted Synthesis, Characterization, and Biological Activity of New Copper (II) Complex with Sulfonamide. Chemistry Proceedings, 14(1), 62. https://doi.org/10.3390/ecsoc-27-16157





