Preparation of a Fluorescent Peptide Substrate to Target Tumor-Associated Macrophages †
Abstract
:1. Introduction
2. Experimental Section
2.1. General
2.2. Methods
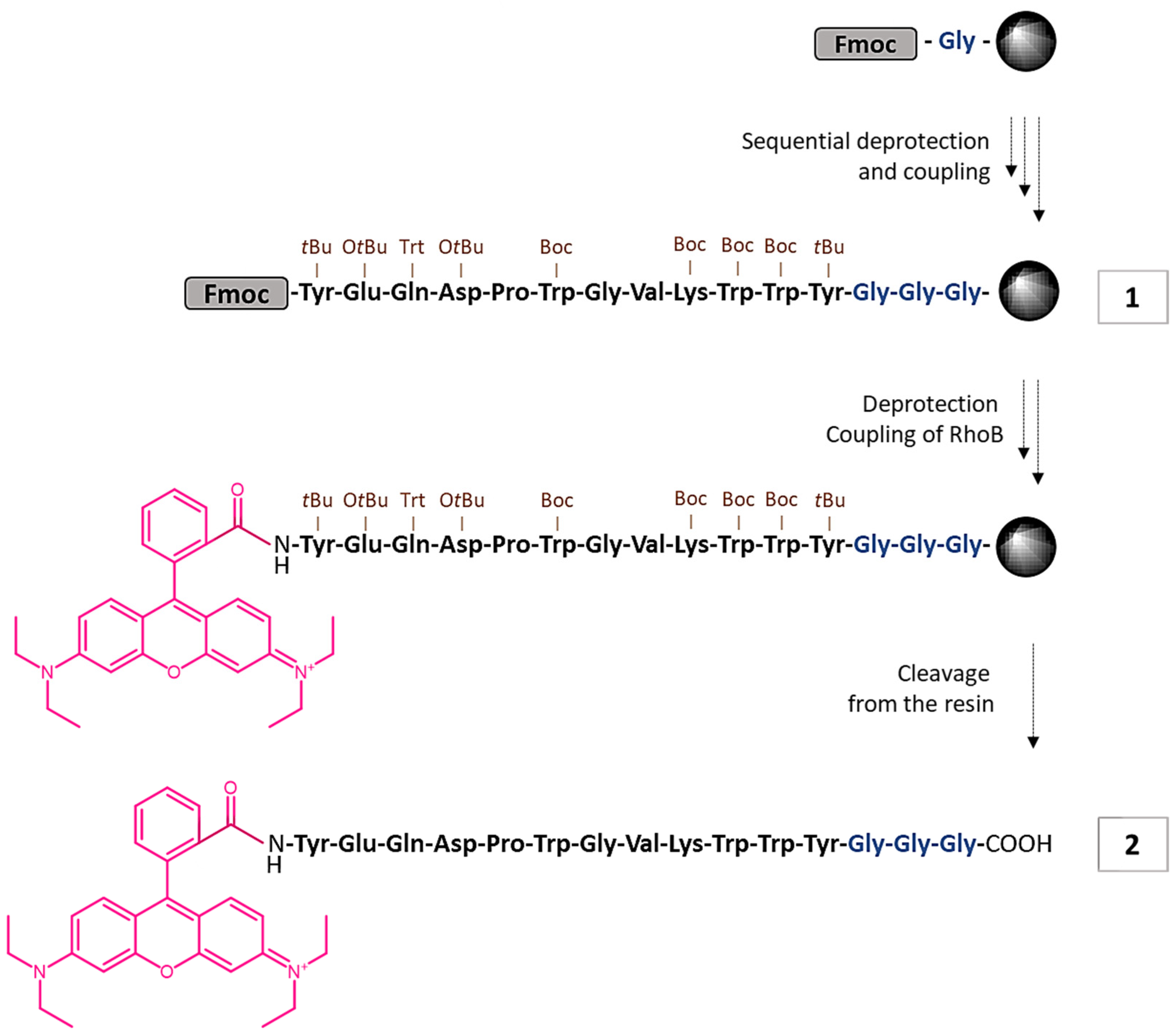
2.2.1. Synthesis of M2pep 1
2.2.2. Synthesis of RhoB-M2pep 2
2.2.3. Photophysical Characterization
3. Results and Discussion
3.1. Synthesis
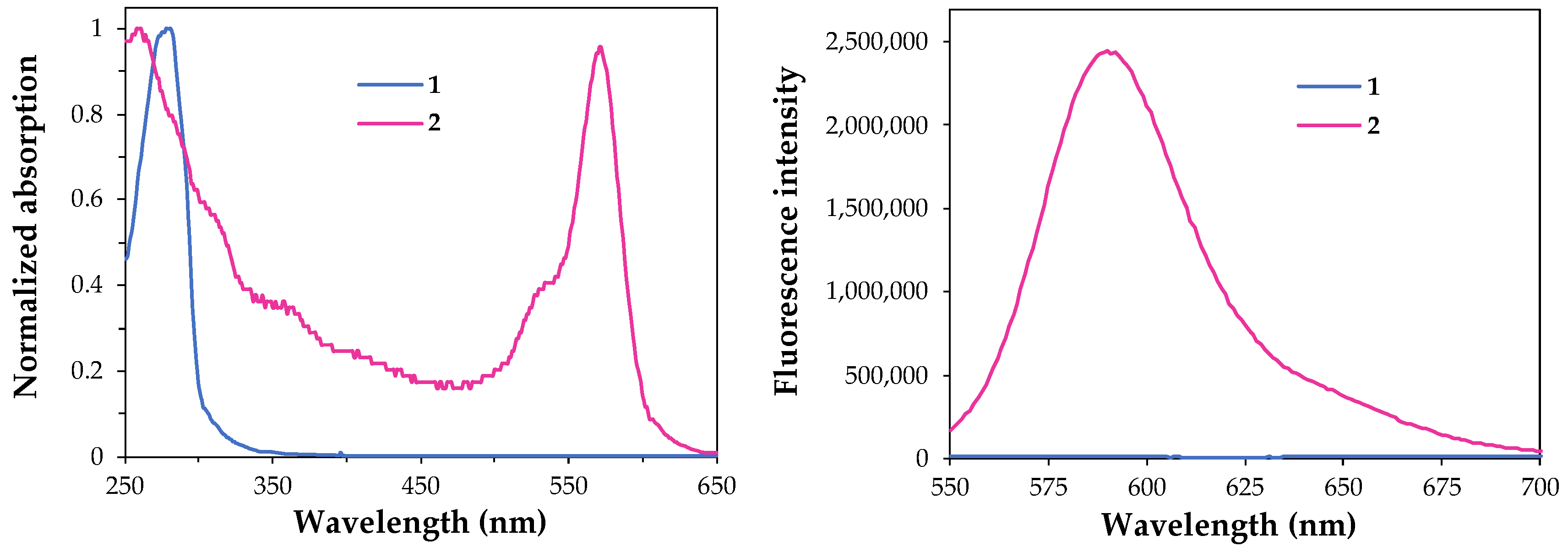
3.2. UV/Vis Absorption and Fluorescence Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, W.; Kapate, N.; Shields, C.W.; Mitragotri, S. Drug Delivery to Macrophages: A Review of Targeting Drugs and Drug Carriers to Macrophages for Inflammatory Diseases. Adv. Drug. Deliv. Rev. 2020, 165–166, 15–40. [Google Scholar] [CrossRef] [PubMed]
- Obeid, E.; Nanda, R.; Fu, Y.-X.; Olopade, O.I. The Role of Tumor-Associated Macrophages in Breast Cancer Progression (Review). Int. J. Oncol. 2013, 43, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Hourani, T.; Holden, J.A.; Li, W.; Lenzo, J.C.; Hadjigol, S.; O’Brien-Simpson, N.M. Tumor Associated Macrophages: Origin, Recruitment, Phenotypic Diversity, and Targeting. Front. Oncol. 2021, 11, 788365. [Google Scholar] [CrossRef] [PubMed]
- Cieslewicz, M.; Tang, J.; Yu, J.L.; Cao, H.; Zavaljevski, M.; Motoyama, K.; Lieber, A.; Raines, E.W.; Pun, S.H. Targeted Delivery of Proapoptotic Peptides to Tumor-Associated Macrophages Improves Survival. Proc. Natl. Acad. Sci. USA 2013, 110, 5919–15924. [Google Scholar] [CrossRef] [PubMed]
- Ngambenjawong, C.; Cieslewicz, M.; Schellinger, J.G.; Pun, S.H. Synthesis and Evaluation of Multivalent M2pep Peptides for Targeting Alternatively Activated M2 Macrophages. J. Control. Release 2016, 224, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, S.; Li, B.; Yang, K.; Lan, M.; Zeng, L. Advances and Perspectives in Carbon Dot-Based Fluorescent Probes: Mechanism, and Application. Coord. Chem. Rev. 2021, 431, 213686. [Google Scholar] [CrossRef]
- Birtalan, E.; Rudat, B.; Kolmel, D.K.; Fritz, D.; Vollrath, S.B.; Schepers, U.; Brase, S. Investigating Rhodamine B-Labeled Peptoids: Scopes and Limitations of Its Applications. Biopolymers 2011, 96, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Belko, N.; Maltanava, H.; Lugovski, A.; Ferreira, R.A.S.; Correia, S.F.H.; Shabunya, P.; Fatykhava, S.; Tabolich, A.; Kulahava, T.; Bahdanava, A.; et al. pH-Sensitive Fluorescent Sensor for Fe(III) and Cu(II) Ions Based on Rhodamine B Acylhydrazone: Sensing Mechanism and Bioimaging in Living Cells. Microchem. J. 2023, 191, 108744. [Google Scholar] [CrossRef]
- Hoenke, S.; Serbian, I.; Deigner, H.-P.; Csuk, R. Mitocanic Di- and Triterpenoid Rhodamine B Conjugates. Molecules 2020, 25, 5443. [Google Scholar] [CrossRef] [PubMed]
- García-Hevia, L.; Casafont, Í.; Oliveira, J.; Terán, N.; Fanarraga, M.L.; Gallo, J.; Bañobre-López, M. Magnetic Lipid Nanovehicles Synergize the Controlled Thermal Release of Chemotherapeutics with Magnetic Ablation While Enabling Non-Invasive Monitoring by MRI for Melanoma Theranostics. Bioact. Mater. 2021, 8, 153–164. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leite, Â.B.M.P.; Martins, C.D.F.; Raposo, M.M.M.; Costa, S.P.G. Preparation of a Fluorescent Peptide Substrate to Target Tumor-Associated Macrophages. Chem. Proc. 2023, 14, 63. https://doi.org/10.3390/ecsoc-27-16101
Leite ÂBMP, Martins CDF, Raposo MMM, Costa SPG. Preparation of a Fluorescent Peptide Substrate to Target Tumor-Associated Macrophages. Chemistry Proceedings. 2023; 14(1):63. https://doi.org/10.3390/ecsoc-27-16101
Chicago/Turabian StyleLeite, Ângela B. M. P., Cátia D. F. Martins, M. Manuela M. Raposo, and Susana P. G. Costa. 2023. "Preparation of a Fluorescent Peptide Substrate to Target Tumor-Associated Macrophages" Chemistry Proceedings 14, no. 1: 63. https://doi.org/10.3390/ecsoc-27-16101
APA StyleLeite, Â. B. M. P., Martins, C. D. F., Raposo, M. M. M., & Costa, S. P. G. (2023). Preparation of a Fluorescent Peptide Substrate to Target Tumor-Associated Macrophages. Chemistry Proceedings, 14(1), 63. https://doi.org/10.3390/ecsoc-27-16101






