Oxidative Aromatization of Some 1,4-Dihydropyridine Derivatives Using Pyritic Ash in Eco-Sustainable Conditions †
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Procedures
2.2. Catalyst for Oxidative Aromatization
2.3. Catalyst for Synthesis of 1,4-DHP (Model Compounds)
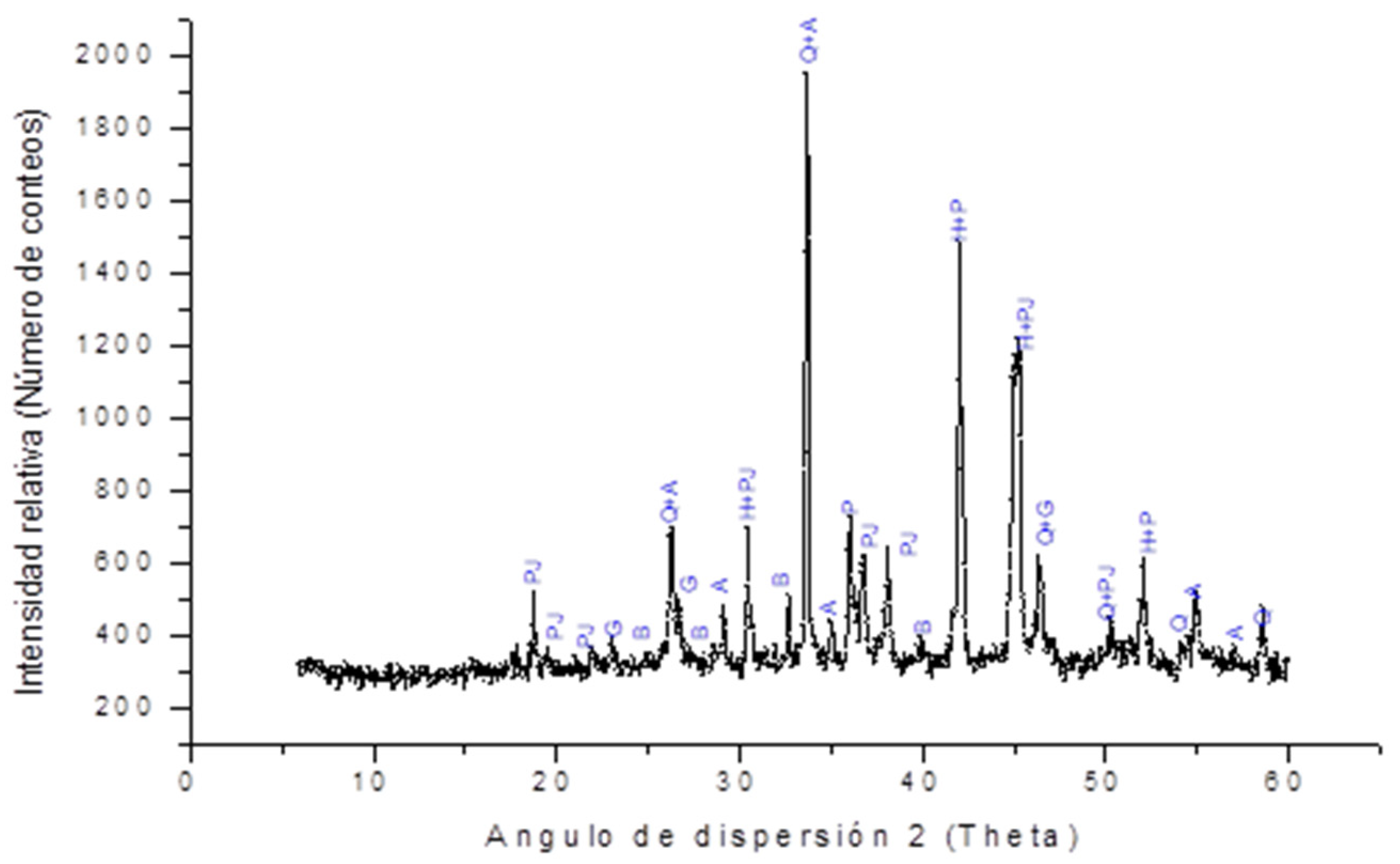
2.4. X-ray Diffraction Analysis. Phase Determination of the Catalyst Used
2.5. Synthesis of 1,4-DHPs (Two Model Compounds)
2.6. Oxidative Aromatization of Model 1,4-DHPs under Catalytic Heterogeneous Condition Using Pyritic Ash. Synthesis of Pyridinic Derivatives
2.7. Gas Chromatography Coupled with Mass Spectrometry (GC-MS)
2.8. NMR Studies
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eisner, U.; Kuthan, J. Chemistry of dihydropyridines. Chem. Rev. 1972, 72, 1–42. [Google Scholar] [CrossRef]
- Kuthan, J.; Kurfurst, A. Development in dihydropyridine chemistry. Ind. Eng. Chem. Prod. Res. Dev. 1982, 21, 191–261. [Google Scholar] [CrossRef]
- Khot, S.; Auti, P.B.; Khedkar, S.A. Diversified Synthetic Pathway of 1, 4-Dihydropyridines: A Class of Pharmacologically Important Molecules. Mini-Rev. Med. Chem. 2021, 2, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Nesterova, E.Y.; Grishchenko, A.A. Main Directions in the Development of 1,4-Dihydropyridine Chemistry—A Literature Review. Bull. Dnipropetr. Univ. Ser. Chem. 2013, 19, 66–88. (In Russian) [Google Scholar]
- Mishra, A.P.; Bajpai, A.; Rai, A.K. 1,4-Dihydropyridine: A Dependable Heterocyclic Ring with the Promising and the Most Anticipable Therapeutic Effects. Mini-Rev. Med. Chem. 2019, 19, 1219–1254. [Google Scholar] [CrossRef]
- Ling, Y.; Hao, Z.-H.; Liang, D.; Zhang, C.L.; Liu, Y.-F.; Wang, Y. The Expanding Role of Pyridine and Dihydropyridine Scaffolds in Drug Design. Drug Des. Dev. Ther. 2012, 15, 4289–4338. [Google Scholar] [CrossRef] [PubMed]
- Sausinsh, A.E.A.; Dubur, G.Y. Synthesis of 1,4-dihydropyridines in cyclocondensation reactions. Chem. Heterocycl. Compd. 1992, 4, 435–467. (In Russian) [Google Scholar]
- Matern, A.I.; Charushin, V.N.; Chupakhin, O.N. Progress in the Studies of Oxidation of Dihydropyridines and Their Analogues. Russ. Chem. Rev. 2007, 76, 1–23. (In Russian) [Google Scholar] [CrossRef]
- Contreras-Cruz, D.A.; Cantú-Reyes, M.; García-Sánchez, J.M.; Peña-Ortíz, D.; Sánchez-Carmona, M.L.; Miranda, L.D. Shedding Blue Light on the Undergraduate Laboratory: An Easy-to-Assemble LED Photoreactor for Aromatization of a 1,4-Dihydropyridine. J. Chem. Educ. 2019, 96, 2015–2020. [Google Scholar] [CrossRef]
- Khaledian, D.; Rostami, A.; Zarei, S.A.; Mohammadi, B. Aerobic oxidative aromatization of Hantzsch 1,4-dihydropyridines via an anomeric-based oxidation in the presence of Laccase enzyme/4-Phenyl urazole as a cooperative catalytic oxidation system. J. Iran. Chem. Soc. 2019, 16, 1871–1878. [Google Scholar] [CrossRef]
- Ray, S.; Brown, M.; Bhaumik, A.; Dutta, A.; Mukhopadhyay, C. A new MCM-41 supported HPF6 catalyst for the library synthesis of highly substituted 1,4-dihydropyridines and oxidation to pyridines: Report of one-dimensional packing towards LMSOMs and studies on their photophysical properties. Green Chem. 2013, 15, 1910–1924. [Google Scholar] [CrossRef]
- Sepehrmansourie, H.; Zarei, M.; Zolfigol, M.A.; Babaee, S.; Rostamnia, S. Application of novel nanomagnetic metal–organic frameworks as a catalyst for the synthesis of new pyridines and 1,4-dihydropyridines via a cooperative vinylogous anomeric based oxidation. Sci. Rep. 2021, 11, 5279–5294. [Google Scholar] [CrossRef] [PubMed]
- Milovanovic, J.; Gündüz, M.G.; Zerva, A.; Petkovic, M.; Beskoski, V.; Thomaidis, N.S.; Topakas, E.; Nikodinovic-Runic, J. Synthesis and Laccase-Mediated Oxidation of New Condensed 1,4-Dihydropyridine Derivatives. Catalysts 2021, 11, 727. [Google Scholar] [CrossRef]
- Abdoli-Senejani, M.; Karami, K. Ultrasound-Assisted Heterogeneous Oxidation of 1,4-Dihydropyridines. Org. Prep. Proced. Int. 2020, 52, 274–281. [Google Scholar] [CrossRef]
- Comins, D.L.; Higuchi, K.; Young, D.W. Dihydropyridine Preparation and Application in the Synthesis of Pyridine Derivatives. In Advances in Heterocyclic Chemistry, 1st ed.; Katritzky, A.R., Ed.; Elsevier: Gainesville, FL, USA, 2013; Volume 110, pp. 175–235. [Google Scholar] [CrossRef]
- Turovska, B.; Goba, I.; Lielpetere, A.; Glezer, V. Electrochemistry of pyridine derivatives. J. Solid State Electrochem. 2023, 27, 1717–1729. [Google Scholar] [CrossRef]
- Zafar, A.M.; Jabeen, M.; Aslam, N.; Anjum, S.; Ghafoor, A.; Khan, M. Oxidation of Some Dihydropyridine Derivatives to Pyridine Via Different Methods. Front. Chem. Sci. 2021, 2, 117–131. [Google Scholar] [CrossRef]
- Grison, C.; Ki, Y.L.T. Ecocatalysis, a new vision of Green and Sustainable Chemistry. Curr. Opin. Green Sustain. Chem. 2021, 29, 100461. [Google Scholar] [CrossRef]
- Rubab, L.; Anum, A.; Al-Hussain, S.A.; Irfan, A.; Ahmad, S.; Ullah, S.; Al-Mutairi, A.A.; Zaki, M.E.A. Green Chemistry in Organic Synthesis: Recent Update on Green Catalytic Approaches in Synthesis of 1,2,4-Thiadiazoles. Catalysts 2022, 12, 1329. [Google Scholar] [CrossRef]
- Gabarrón, M.; Babur, O.; Soriano-Disla, J.M.; Faz, A.; Acosta, J.A. Composition and risk assessment of roasted pyrite ash from fertilizer production. Chemosphere 2018, 209, 209–277. [Google Scholar] [CrossRef]
- Oliveira, M.L.; Ward, C.R.; Izquierdo, M.; Sampaio, C.H.; de Brum, I.A.; Kautzmann, R.M.; Sabedot, S.; Querol, X.; Silva, L.F. Chemical composition and minerals in pyrite ash of an abandoned sulfuric acid production plant. Sci. Total Environ. 2012, 430, 34–47. [Google Scholar] [CrossRef]
- Ramos-Guivar, J.R.; Zarria-Romero, J.Y.; Castro-Merino, I.L.; Greneche, J.M.; Passamani, E. Improvement of the thermal stability of nanomaghemite by functionalization with type 5A zeolite and magnetic properties studied by in-field 57Fe Mössbauer measurements. J. Magn. Magn. Mater. 2022, 552, 169241. [Google Scholar] [CrossRef]
- Flores, D. Synthesis and Structural and Vibrational Characterization of Maghemite Nanoparticles Functionalized with Hexadecyltrimethylammonium Bromide and Zeolite Type 5A. Ph.D. Thesis, Faculty of Physical Sciences, Universidad Nacional Mayor de San Marcos, Lima, Peru, 2022. (In Spanish). [Google Scholar]
- Tacoronte Morales, J.E. Prometheus Project of the Republic of Ecuador. National Secretary of Science, Technology and Innovation, 2013–2018. SENESCyT-UCE-09085060. Development of Catalytic Processes under Eco-Sustainable Conditions. (In Spanish)
- Widayat, D.; Putra, A.; Nursafitri, I. Synthesis and catalytic evaluation of hematite (α-Fe2O3) magnetic nanoparticles from iron sand for waste cooking oil conversion to produce biodiesel through esterification-transesterification method. IOP Conf. Ser. Mater. Sci. Eng. 2020, 509, 012035. [Google Scholar] [CrossRef]
- Khalil, M.; Liu, N.; Lee, R.L. Catalytic Aqua-thermolysis of Heavy Crude Oil Using Surface-Modified Hematite Nanoparticles. Ind. Eng. Chem. Res. 2017, 56, 4572–4579. [Google Scholar] [CrossRef]
- Gholinejad, M.; Shojafar, M.; Sansano, J.M. Enhanced catalytic activity of natural hematite-supported ppm levels of Pd in nitroarenes reduction. J. Iran Chem. Soc. 2020, 17, 2033–2043. [Google Scholar] [CrossRef]
- Galadima, A.; Muraza, O. Waste materials for production of biodiesel catalysts: Technological status and prospects. J. Clean. Prod. 2020, 263, 121358. [Google Scholar] [CrossRef]


| Chemical Composition | % (m/m) |
|---|---|
| Total Iron | 40.97 |
| Acid Soluble Iron | 37.09 |
| Ferrous Iron | 34.75 |
| Ferrous + metallic iron | 2.34 |
| Water soluble iron | 0.09 |
| Total Sulfur | 1.84 |
| Unroasted Sulfur | 1.41 |
| Sulfur by aqueous extraction | 0.20 |
| Total Lead | 3.50 |
| Total Copper | 0.20 |
| Leachable Copper | 0.20 |
| Total Zn | 0.41 |
| Leachable Zn | 0.00 |
| Silica | 21.15 |
| Manganese | 0.08 |
| Barium | 5.85 |
| Potassium | 0.38 |
| Magnesium | 0.18 |
| Moisture | 1.44 |
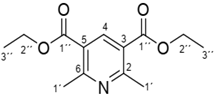
| Structure | Molecular Characterization 1 |
|---|---|
 | Yield: 64%. M.P.: 169–171 °C (lit. 178–183 °C). NMR 1H δ (250 MHz, CDCl3): δ = 5.13 (s, 1H); 4.17 (qua, J = 7 Hz, 2″-4H); 3.17 (s, 4-2H); 2.19 (s, 1′-6H); 1.29 (t, J = 7 Hz, 3″-6H) ppm.; GC-MS: 252.9 (M+.); 224.3(100%); 207.2; 196.2; 178.5; 151.5; 105.6 m/z. |
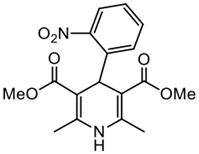 | 1.- Diethyl-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate Yield: 76%. M.P: 165–167 °C (lit. 172–174 °C). NMR 1H (250 MHz, CDCl3): δ = 7.68 (d, J = 8 Hz, 1H); 7.49 (dd, J = 6 Hz, 2H); 7.25 (td, J = 8 Hz, 1H); 5.77 (s, 1H); 5.72 (s, 1H); 3.59 (s, 6H);2.34 (s, 6H) ppm. NMR 13C (40 MHz, CDCl3): δ = 167.49; 147.88; 144.76; 142.07; 132.68; 131.03; 127.02; 123.88; 103.71; 50.99; 34.54; 19.50 ppm. Nife.- Dimethyl 4-(2-nitrophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate |
| Structure | Molecular Characterization 1 |
|---|---|
 | Yield: 83%. M.P.: 65–67 °C (lit. 70–72 °C). NMR 1H (250 MHz, CDCl3): 8.68 (s, 1H, 4-H); 4.40 (qua, J = 4 Hz, 4H, 2”-H); 2.85 (s, 6H, 1′-H); 1.42 (t, J = 4 Hz, 6H, 3”-H); ppm. GC-MS: m/z: 251.5 (M+.); 206.1 (100%); 178.5; 151.1; 106.5. |
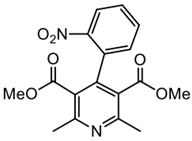 | 1-ox. Diethyl-2,6-dimethyl-pyridine-3,5-dicarboxylate Yield: 96%. M.P.: 98–100 °C (lit. 105 °C). NMR 1H (250 MHz, CDCl3): 8.21 (dd, J = 8 Hz, 1H, CH); 8.12 (d, J = 8 Hz, 1H, CH); 7.61 (m, 4H, CH); 7.21 (m, 1H, CH); 6.92 (s, 1H, CH); 3.54 (s,3H, OCH3); 3.50 (s, 3H, OCH3); 2.65 (s, 6H, CH3); 2.58 (s, 3H, CH3) ppm. NMR. 13C (40 MHz, CDCl3): 167.9 (C=O ester); 167.3 (C=O ester); 159.2; 157.1; 156.4; 147.8; 147.6; 146.5; 134.1 (C-C); 132.9; 130.9; 130.6; 129.6; 129.2; 124.4; 124.3; 120.6 (CH); 52.2 (OCH3); 52.0 (OCH3);24.5 (CH3); 23.7 (CH3) ppm. GC-MS: m/z = 281.9 (M+.); 280.9; 208.2; 206.8 (100%); 198.1.Nife-ox. Dimethyl 4-(2-nitrophenyl)-2,6-dimethyl-pyridine-3,5-dicarboxylate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales, J.E.T.; Villavicencio, C.B.; Cervantes, X.L.G.; Canchingre, M.E.; Pedroso, M.T.C. Oxidative Aromatization of Some 1,4-Dihydropyridine Derivatives Using Pyritic Ash in Eco-Sustainable Conditions. Chem. Proc. 2023, 14, 61. https://doi.org/10.3390/ecsoc-27-16066
Morales JET, Villavicencio CB, Cervantes XLG, Canchingre ME, Pedroso MTC. Oxidative Aromatization of Some 1,4-Dihydropyridine Derivatives Using Pyritic Ash in Eco-Sustainable Conditions. Chemistry Proceedings. 2023; 14(1):61. https://doi.org/10.3390/ecsoc-27-16066
Chicago/Turabian StyleMorales, Juan Enrique Tacoronte, Carla Bernal Villavicencio, Xavier Leopoldo Gracia Cervantes, Maria Elizabeth Canchingre, and Maria Teresa Cabrera Pedroso. 2023. "Oxidative Aromatization of Some 1,4-Dihydropyridine Derivatives Using Pyritic Ash in Eco-Sustainable Conditions" Chemistry Proceedings 14, no. 1: 61. https://doi.org/10.3390/ecsoc-27-16066
APA StyleMorales, J. E. T., Villavicencio, C. B., Cervantes, X. L. G., Canchingre, M. E., & Pedroso, M. T. C. (2023). Oxidative Aromatization of Some 1,4-Dihydropyridine Derivatives Using Pyritic Ash in Eco-Sustainable Conditions. Chemistry Proceedings, 14(1), 61. https://doi.org/10.3390/ecsoc-27-16066






