Synthesis of New Representatives of Push–Pull Enamines 5-Aryl-3-((dimethylamino)methylene)furan-2(3H)-Ones †
Abstract
:1. Introduction
2. Results and Discussion
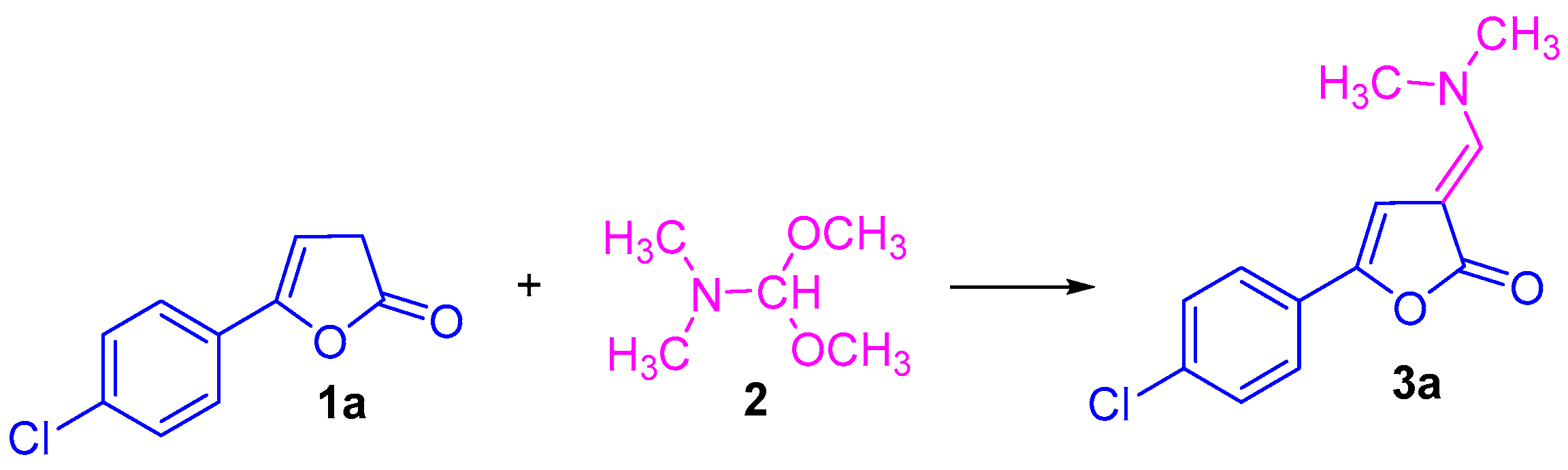
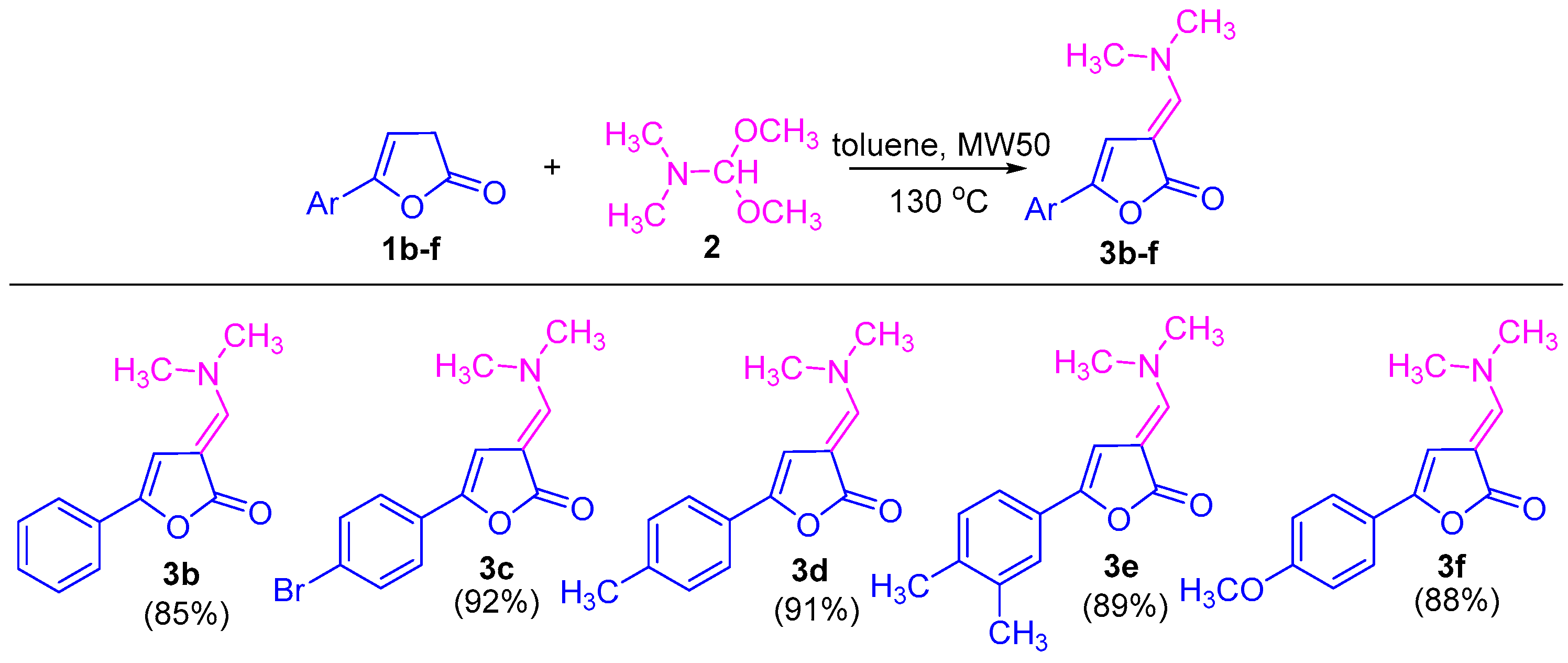
2.1. Synthesis of 5-Aryl-substituted 3-((dimethylamino)methylene)furan-2(3H)-Ones
2.2. Structure of 3-Dimethylaminomethylene Derivatives of Furan-2(3H)-Ones 3a–f
3. Material and Methods
3.1. Physical Measurements
3.2. Synthesis and Characterization of Compounds 3a–f
- 5-(4-(Chlorophenyl)-3-((dimethylamino)methylene)furan-2(3H)-one 3a
- 5-Phenyl-3-((dimethylamino)methylene)furan-2(3H)-one 3b
- 5-(4-(Bromophenyl)-3-((dimethylamino)methylene)furan-2(3H)-one 3c
- 5-(4-(Methylphenyl)-3-((dimethylamino)methylene)furan-2(3H)-one 3d
- 5-(3,4-(Dimethylphenyl)-3-((dimethylamino)methylene)furan-2(3H)-one 3e
- 5-(4-(Methoxyphenyl)-3-((dimethylamino)methylene)furan-2(3H)-one 3f
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdel-Hamid, H.F.; Soliman, A.; Helaly, F.M.; Ragab, S. Cytotoxic potency and induced biochemical parameters in mice serum of new furan derivatives against liver cancer cell line. Acta Pol. Pharm. 2011, 68, 499–505. [Google Scholar] [PubMed]
- Sicak, Y. Design and antiproliferative and antioxidant activities of furan-based thiosemicarbazides and 1,2,4-triazoles: Their structure-activity relationship and SwissADME predictions. Med. Chem. Res. Lett. 2021, 30, 1557–1568. [Google Scholar] [CrossRef]
- Vicidomini, C.; Cioffi, F.; Broersen, K.; Roviello, V.; Riccardi, C.; Montesarchio, D.; Capasso, D.; Di Gaetano, S.; Musumeci, D.; Roviello, G. Benzodifurans for biomedical applications: BZ4, a selective anti-proliferative and anti-amyloid lead compound. Future Med. Chem. 2019, 11, 285–302. [Google Scholar] [CrossRef] [PubMed]
- Kwiecien, H.; Peruzynska, M.; Stachowicz, K.; Piotrowska, K.; Bujak, I.; Kopytko, P.; Droździk, M. Synthesis and biological evaluation of 3-functionalized 2-phenyl- and 2-alkylbenzo[b]furans as antiproliferative agents against human melanoma cell line. Bioorg. Chem. 2019, 88, 102930–102941. [Google Scholar] [CrossRef] [PubMed]
- Mehta, D.K.; Das, R. Synthesis and in- vitro antioxidant activity of some new 2,5-disubstituted-1,3,4-oxadiazoles containing furan moiety. Int. J. Pharm. Sci. Res. 2011, 2, 2959–2963. [Google Scholar]
- Donlawson, C.; Nweneka, D.O.; Orie, K.; Okah, R. Synthesis and bioactivity of 1-((2-carbamoylguanidino)(furan-2-ylmethyl)urea. Am. J. Anal. Chem. 2020, 11, 280–288. [Google Scholar] [CrossRef]
- Xia, L.; Idhayadhulla, A.; Lee, Y.R.; Wee, Y.-J.; Kim, S.H. Anti-tyrosinase, antioxidant, and antibacterial activities of novel 5-hydroxy-4-acetyl-2,3-dihydronaphtho [1,2-b]furans. Eur. J. Med. Chem. 2014, 86, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Malladi, S.; Nadh, V.; Suresh Babu, K.; Suri Babu, P. Synthesis and antibacterial activity studies of 2,4-disubstituted furan derivatives. Beni-Suef Univ. J. Basic Appl. Sci. 2017, 6, 345–353. [Google Scholar] [CrossRef]
- El-Borai, M.A.; Rizk, H.F.; Beltagy, D.M.; El-Deeb, I.Y. Microwave-assisted synthesis of some new pyrazolopyridines and their antioxidant, antitumor and antimicrobial activities. Eur. J. Med. Chem. 2013, 66, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Farghaly, T.A.; Abdel Hafez, N.A.; Ragab, E.A.; Awad, H.M.; Abdalla, M.M. Synthesis, anti-HCV, antioxidant, and peroxynitrite inhibitory activity of fused benzosuberone derivatives. Eur. J. Med. Chem. 2010, 45, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Dai, C.; Jamison, T.F.; Jensen, K.F. A rapid total synthesis of ciprofloxacin hydrochloride in continuous flow. Angew. Chem. Int. Ed. 2017, 56, 8870–8873. [Google Scholar] [CrossRef] [PubMed]
- Abu-Shanab, F.A.; Redhouse, A.D.; Thompson, J.R.; Wakefield, B.J. Synthesis of 2,3,5,6-tetrasubstituted pyridines from enamines derived from N,N-dimethylformamide dimethyl acetal. Synthesis 1995, 1995, 557–560. [Google Scholar] [CrossRef]
- Abu-Shanab, F.A.; Aly, F.M.; Wakefield, B.J. Synthesis of substituted nicotinamides from enamines derived from N,N-dimethylformamide dimethyl acetal. Synthesis 1995, 1995, 923–925. [Google Scholar] [CrossRef]
- Abu-Shanab, F.A.; Elkholy, Y.M.; Elnagdi, M.H. Enaminones as building blocks in organic synthesis: Synthesis of new polyfunctional pyridines, condensed pyridines, and penta substituted benzene. Synth. Commun. 2002, 32, 3493–3502. [Google Scholar] [CrossRef]
- Abu-Shanab, F.A.; Hessen, A.M.; Mousa, S.A.S. Dimethylformamide dimethyl acetal in heterocyclic synthesis: Synthesis of polyfunctionally substituted pyridine derivatives as precursors to bicycles and polycycles. J. Heterocycl. Chem. 2007, 44, 787–791. [Google Scholar] [CrossRef]
- Gupton, J.T.; Krumpe, K.E.; Burnham, B.S.; Dwornik, K.A.; Petrich, S.A.; Du, K.X.; Sikorski, J.A. The application of disubstituted vinylogous iminium salts and related synthons to the regiocontrolled preparation of unsymmetrical 2,3,4-trisubstituted pyrroles. Tetrahedron 1998, 54, 5075–5088. [Google Scholar] [CrossRef]
- Al-Mousawi, S.; John, E.; Al-Kandery, N. Studies with enaminones: Synthesis and chemical reactivity of 2-(4-dimethylamino-2-oxobut-3-enyl)-isoindole-1,3-dione and of 4-(4-dimethylamino-2-oxobut-3-enyloxy)-2H-phthalazin-1-one. J. Heterocycl. Chem. 2004, 41, 381–385. [Google Scholar] [CrossRef]
- Al-Mousawi, S.; John, E.; Abdelkhalik, M.M.; Elnagdi, M.H. Enaminones as building blocks in heterocyclic syntheses: A new approach to polyfunctionally substituted cyclohexenoazines. J. Heterocycl. Chem. 2003, 40, 689–695. [Google Scholar] [CrossRef]
- Bruno, O.; Schenone, S.; Ranise, A.; Bondavalli, F.; Filippelli, W.; Falcone, G.; Mazzeo, F. Antiinflammatory agents: New series of N-substituted amino acids with complex pyrimidine structures endowed with antiphlogistic activity. Il Farmaco 1999, 54, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Mustazza, C.; Giudice, M.R.D.; Borioni, A.; Gatta, F. Synthesis of pyrazolo [1,5-a]-, 1,2,4-triazolo [1,5-a]- and imidazo [1,2-a]pyrimidines related to zaleplon, a new drug for the treatment of insomnia. J. Heterocycl. Chem. 2001, 38, 1119–1129. [Google Scholar] [CrossRef]
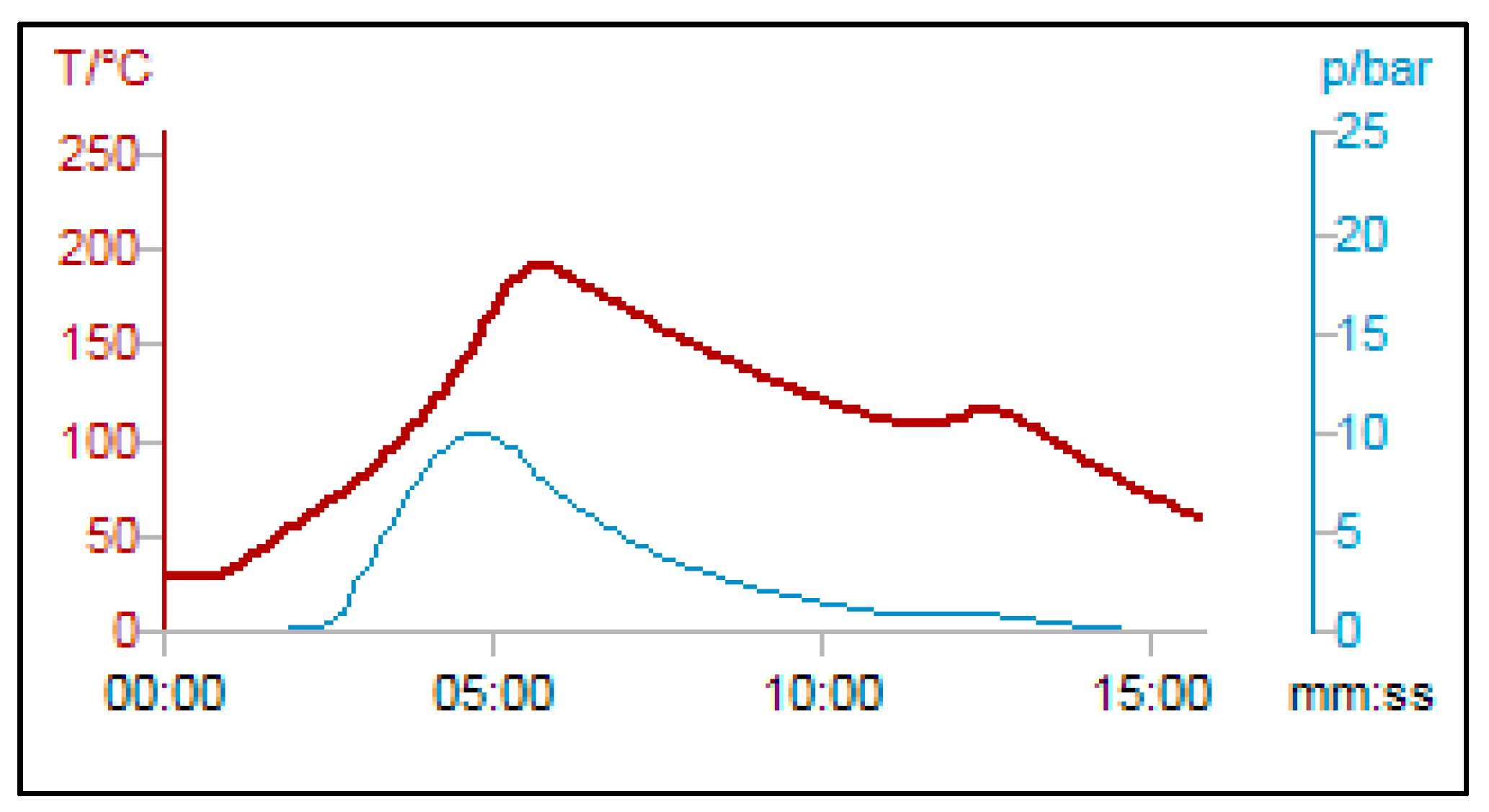
| Entry | Solvent | Temperature, °C | Time (min) | Yield, % |
|---|---|---|---|---|
| 1 | EtOH | 78 | 180 | 50 |
| 2 | i-PrOH | 82 | 178 | 50 |
| 3 | MeCN | 81 | 150 | 50 |
| 4 | 1,4-Dioxane | 101 | 160 | 61 |
| 5 | Benzene | 80 | 173 | 57 |
| 6 | Toluene | 110 | 110 | 70 |
| 7 | Solvent-free | 153 | - | - |
| 8 | Toluene | 115 | 25 | 75 |
| 9 | Toluene | 130 | 6 | 90 |
| 10 | Toluene | 150 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tikhomolova, A.S.; Mamleeva, Z.V.; Yegorova, A.Y. Synthesis of New Representatives of Push–Pull Enamines 5-Aryl-3-((dimethylamino)methylene)furan-2(3H)-Ones. Chem. Proc. 2023, 14, 5. https://doi.org/10.3390/ecsoc-27-16056
Tikhomolova AS, Mamleeva ZV, Yegorova AY. Synthesis of New Representatives of Push–Pull Enamines 5-Aryl-3-((dimethylamino)methylene)furan-2(3H)-Ones. Chemistry Proceedings. 2023; 14(1):5. https://doi.org/10.3390/ecsoc-27-16056
Chicago/Turabian StyleTikhomolova, Alexandra S., Zhanna V. Mamleeva, and Alevtina Yu. Yegorova. 2023. "Synthesis of New Representatives of Push–Pull Enamines 5-Aryl-3-((dimethylamino)methylene)furan-2(3H)-Ones" Chemistry Proceedings 14, no. 1: 5. https://doi.org/10.3390/ecsoc-27-16056
APA StyleTikhomolova, A. S., Mamleeva, Z. V., & Yegorova, A. Y. (2023). Synthesis of New Representatives of Push–Pull Enamines 5-Aryl-3-((dimethylamino)methylene)furan-2(3H)-Ones. Chemistry Proceedings, 14(1), 5. https://doi.org/10.3390/ecsoc-27-16056





