Uranyl Acetate, a Lewis Acid Catalyst for Acetoxylation of Monoterpenic and Steroidal Alcohols †
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Procedures
2.2. Preparation of the Catalyst
Precautionary Measures
- –
- Reducing the amount of material handled as much as possible.
- –
- Not exceeding a working temperature of 200 °C to avoid the thermal decomposition of uranyl acetate.
- –
- Containing unsealed sources to prevent contamination.
- –
- Maintaining a high level of cleanliness.
- –
- Not disposing of uranyl acetate as ordinary waste.
2.3. Acetylation Procedure
3. Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Riemenscheider, W. Ulmann’s Encyclopedia of Industrial Chemistry, 5th ed.; Verlag Chemie: Weinheim, Germany, 1997; Volume A9, p. 565. [Google Scholar]
- Hoffe, G.; Steglich, V.; Vorbruggen, H. 4-Dialkylaminopyridines as Highly Active Acylation Catalysts. Angew. Chem. Int. Ed. Eng. 1978, 17, 569–583. [Google Scholar] [CrossRef]
- Scriven, E.F.V. 4-Dialkylaminopyridines: Super acylation and alkylation catalysts. Chem. Soc. Rev. 1983, 12, 129–161. [Google Scholar] [CrossRef]
- Pasricha, S.; Rangarajan, T.M. Green Acetylation of Primary Aromatic Amines. Resonance 2023, 28, 325–331. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Z.; Liu, M.; Liu, H.; Li, Q.; Xiang, J.; Wu, T.; Han, B. Acylation of phenols to phenolic esters with organic salts. Green Chem. 2022, 24, 9763–9771. [Google Scholar] [CrossRef]
- Rahmatpour, A.; Alinejad, S.; Donyapeyma, G. Noncross-linked polystyrene nanoencapsulation of ferric chloride: A novel and reusable heterogeneous macromolecular Lewis’s acid catalyst toward selective acetylation of alcohols, phenols, amines, and thiols. J. Organomet. Chem. 2022, 961, 122264. [Google Scholar] [CrossRef]
- Sartori, G.; Ballini, R.; Bigi, F.; Bosica, G.; Maggi, R.; Righi, P. Protection (and deprotection) of functional groups in organic synthesis by heterogeneous catalysis. Chem. Rev. 2004, 104, 199–250. [Google Scholar] [CrossRef] [PubMed]
- Valentini, F.; Galloni, P.; Brancadoro, D.; Conte, V.; Sabuzi, F. A Stoichiometric Solvent-Free Protocol for Acetylation Reactions. Front. Chem. 2022, 10, 842190. [Google Scholar] [CrossRef] [PubMed]
- Chutia, R.; Chetia, B. Acetylation of alcohols, phenols and amines using waste plant extract. SN Appl. Sci. 2020, 2, 1564–1571. [Google Scholar] [CrossRef]
- Mayr, S.; Zipse, H. Annelated Pyridine Bases for the Selective Acylation of 1,2-Diols. Eur. J. Org. Chem. 2022, 29, e202101521. [Google Scholar] [CrossRef]
- Peng, P.; Linseis, M.; Winter, R.F.; Schmidt, R.R. Regioselective Acylation of Diols and Triols: The Cyanide Effect. J. Am. Chem. Soc. 2016, 138, 6002–6009. [Google Scholar] [CrossRef]
- Estevão, M.S.; Afonso, C.A.M. Effect of a catalyst in the Acylation of Alcohols with Acetic Anhydride: Manipulation of a natural aroma. In Comprehensive Organic Chemistry Experiments for the Laboratory Classroom; Royal Society of Chemistry: London, UK, 2016; pp. 169–171. [Google Scholar]
- Choudary, B.M.; Reddy, P.N. Selective Markovnikov’s addition of trifluoroacetic acid to alkenes using vanadium(V) oxide. J. Chem. Soc. Chem. 1993, 4, 405–407. [Google Scholar] [CrossRef]
- Collis, A.E.; Horváth, I.T. Heterogenization of homogeneous catalytic systems. Catal. Sci. Technol. 2011, 1, 912–919. [Google Scholar] [CrossRef]
- Sharma, R.; Kabra, B.; Vaidya, V. Photochemical oxidation of 2-imidazolidinethione by uranyl acetate. Asian J. Chem. 1996, 8, 500–504. [Google Scholar]
- Selishchev, D.S.; Filippov, T.N.; Lyulyukin, M.N.; Kozlov, D.V. Uranyl-modified TiO2 for complete photocatalytic oxidation of volatile organic compounds under UV and visible light. Chem. Eng. J. 2019, 370, 1440–1449. [Google Scholar] [CrossRef]
- Hu, D.; Jiang, X. Stepwise benzylic oxygenation via uranyl-photocatalysis. Green Chem. 2022, 24, 124–129. [Google Scholar] [CrossRef]

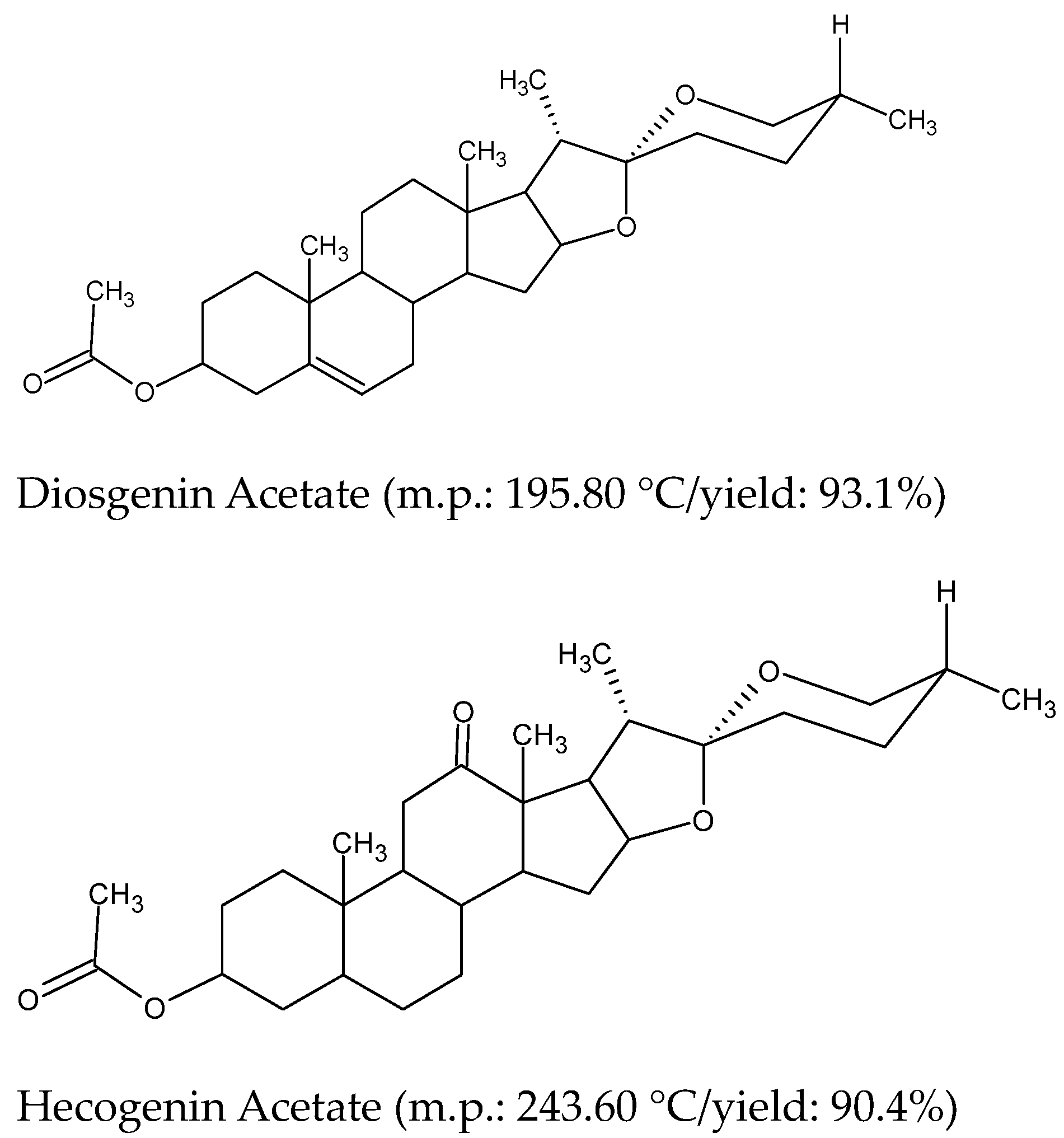
| Entry | Substrate | O-Acetoxy-Derivative a |
|---|---|---|
| 1 | 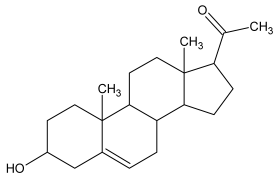 Pregnenolone | 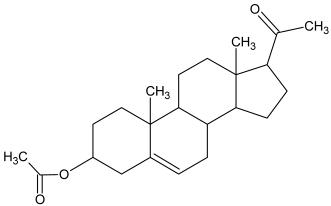 Pregnenolone acetate (m.p:191.7 °C/96.8%) |
| 2 |  Estrone | 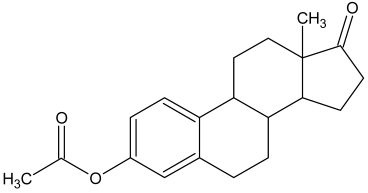 Estrone acetate (m.p.: 129.7 °C/97.3%) |
| 3 | 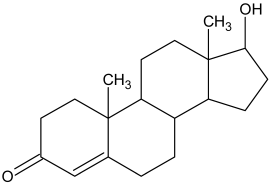 Δ-4-androsten-17β-ol | 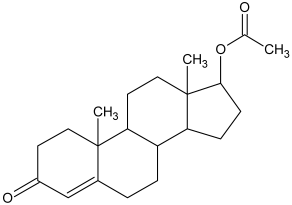 Δ-4-androsten-17β-ol-acetate (m.p.: 142.6 °C/83.4%) |
| 4 | (−)-Menthol | Menthyl acetate (b.p.: 57.8 °C/82%) |
| 5 | (+/−)-endo-Norborneol | Norbornyl acetate (b.p.: 202 °C/85%) |
| 6 | α-Terpineol | (±)-α-Terpinyl acetate (b.p: > 185 °C) b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales, J.E.T.; Villavicencio, C.B.; Cervantes, X.L.G.; Canchingre, M.E.; Pedroso, M.T.C. Uranyl Acetate, a Lewis Acid Catalyst for Acetoxylation of Monoterpenic and Steroidal Alcohols. Chem. Proc. 2023, 14, 44. https://doi.org/10.3390/ecsoc-27-16064
Morales JET, Villavicencio CB, Cervantes XLG, Canchingre ME, Pedroso MTC. Uranyl Acetate, a Lewis Acid Catalyst for Acetoxylation of Monoterpenic and Steroidal Alcohols. Chemistry Proceedings. 2023; 14(1):44. https://doi.org/10.3390/ecsoc-27-16064
Chicago/Turabian StyleMorales, Juan Enrique Tacoronte, Carla Bernal Villavicencio, Xavier Leopoldo Gracia Cervantes, Maria Elizabeth Canchingre, and Maria Teresa Cabrera Pedroso. 2023. "Uranyl Acetate, a Lewis Acid Catalyst for Acetoxylation of Monoterpenic and Steroidal Alcohols" Chemistry Proceedings 14, no. 1: 44. https://doi.org/10.3390/ecsoc-27-16064
APA StyleMorales, J. E. T., Villavicencio, C. B., Cervantes, X. L. G., Canchingre, M. E., & Pedroso, M. T. C. (2023). Uranyl Acetate, a Lewis Acid Catalyst for Acetoxylation of Monoterpenic and Steroidal Alcohols. Chemistry Proceedings, 14(1), 44. https://doi.org/10.3390/ecsoc-27-16064






