Effects of N-(Alkoxyphenyl)-1-hydroxynaphthalene-2-carboxamides on Intestinal Microbial Communities †
Abstract
:1. Introduction
2. Materials and Methods
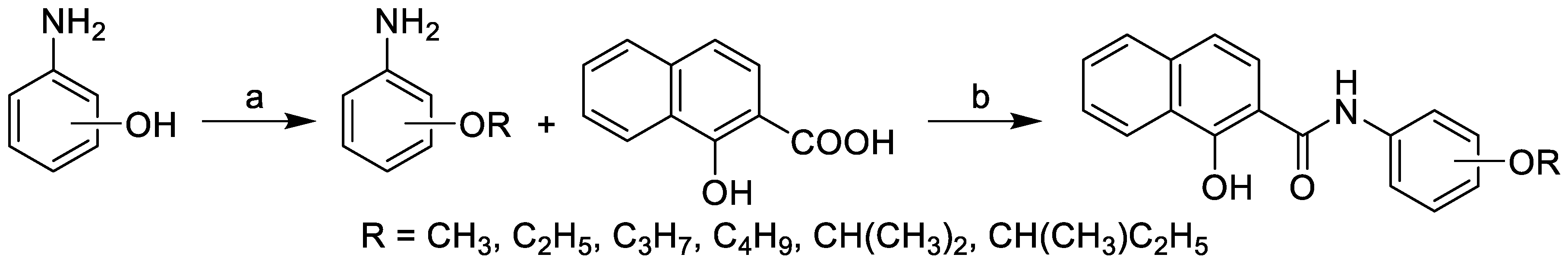
2.1. Synthesis
2.2. In Vitro Antibacterial Susceptibility Testing
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dahal, P. Enterobacteriaceae—Definition, Characteristics, Identification. Microbe Notes. 2023. Available online: https://microbenotes.com/enterobacteriaceae (accessed on 22 September 2023).
- Axelrad, J.E.; Cadwell, K.H.; Colombel, J.F.; Shah, S.C. The role of gastrointestinal pathogens in inflammatory bowel disease: A systematic review. Therap. Adv. Gastroenterol. 2021, 14, 17562848211004493. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, F.; Xue, J.; Lee, S.A.; Liu, L.; Riordan, S.M. Bacterial species associated with human inflammatory bowel disease and their pathogenic mechanisms. Front. Microbiol. 2022, 13, 801892. [Google Scholar] [CrossRef] [PubMed]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhao, W.; Lan, P.; Mou, X. The microbiome in inflammatory bowel diseases: From pathogenesis to therapy. Protein Cell. 2021, 12, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Niclosamide–DrugBank. Available online: https://go.drugbank.com/drugs/DB06803 (accessed on 22 September 2023).
- Jiang, H.; Li, A.M.; Ye, J. The magic bullet: Niclosamide. Front. Oncol. 2022, 12, 1004978. [Google Scholar] [CrossRef] [PubMed]
- Zadrazilova, I.; Pospisilova, S.; Masarikova, M.; Imramovsky, A.; Ferriz, J.M.; Vinsova, J.; Cizek, A.; Jampilek, J. Salicylanilide carbamates: Promising antibacterial agents with high in vitro activity against methicillin-resistant Staphylococcus aureus (MRSA). Eur. J. Pharm. Sci. 2015, 77, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Copp, J.N.; Pletzer, D.; Brown, A.S.; Van der Heijden, J.; Miton, C.M.; Edgar, R.J.; Rich, M.H.; Little, R.F.; Williams, E.M.; Hancock, R.E.W.; et al. Mechanistic understanding enables the rational design of salicylanilide combination therapies for Gram-negative infections. mBio 2020, 11, e02068-20. [Google Scholar] [CrossRef]
- Kushkevych, I.; Kollar, P.; Ferreira, A.L.; Palma, D.; Duarte, A.; Lopes, M.M.; Bartos, M.; Pauk, K.; Imramovsky, A.; Jampilek, J. Antimicrobial effect of salicylamide derivatives against intestinal sulfate-reducing bacteria. J. Appl. Biomed. 2016, 14, 125–130. [Google Scholar] [CrossRef]
- Kauerova, T.; Perez-Perez, M.J.; Kollar, P. Salicylanilides and their anticancer properties. Int. J. Mol. Sci. 2023, 24, 1728. [Google Scholar] [CrossRef]
- Pindjakova, D.; Pilarova, E.; Pauk, K.; Michnova, H.; Hosek, J.; Magar, P.; Cizek, A.; Imramovsky, A.; Jampilek, J. Study of biological activities and ADMET-related properties of salicylanilide-based peptidomimetics. Int. J. Mol. Sci. 2022, 23, 11648. [Google Scholar] [CrossRef]
- Gonec, T.; Kos, J.; Zadrazilova, I.; Pesko, M.; Keltosova, S.; Tengler, J.; Bobal, P.; Kollar, P.; Cizek, A.; Kralova, K.; et al. Antimycobacterial and herbicidal activity of ring-substituted 1-hydroxynaphthalene-2-carboxanilides. Bioorg. Med. Chem. 2013, 21, 6531–6541. [Google Scholar] [CrossRef] [PubMed]
- Gonec, T.; Pospisilova, S.; Kauerova, T.; Kos, J.; Dohanosova, J.; Oravec, M.; Kollar, P.; Coffey, A.; Liptaj, T.; Cizek, A.; et al. N-Alkoxyphenylhydroxynaphthalenecarboxamides and their antimycobacterial activity. Molecules 2016, 21, 1068. [Google Scholar] [CrossRef] [PubMed]
- Michnova, H.; Pospisilova, S.; Gonec, T.; Kapustikova, I.; Kollar, P.; Kozik, V.; Musiol, R.; Jendrzejewska, I.; Vanco, J.; Travnicek, Z.; et al. Bioactivity of methoxylated and methylated 1-hydroxynaphthalene-2-carboxanilides: Comparative molecular surface analysis. Molecules 2019, 24, 2991. [Google Scholar] [CrossRef] [PubMed]
- Gonec, T.; Pindjakova, D.; Vrablova, L.; Strharsky, T.; Michnova, H.; Kauerova, T.; Kollar, P.; Oravec, M.; Jendrzejewska, I.; Cizek, A.; et al. Antistaphylococcal activities and ADME-related properties of chlorinated arylcarbamoylnaphthalenylcarbamates. Pharmaceuticals 2022, 15, 715. [Google Scholar] [CrossRef] [PubMed]
- Spaczynska, E.; Mrozek-Wilczkiewicz, A.; Malarz, K.; Kos, J.; Gonec, T.; Oravec, M.; Gawecki, R.; Bak, A.; Dohanosova, J.; Kapustikova, I.; et al. Design and synthesis of anticancer 1-hydroxynaphthalene-2-carboxanilides with a p53 independent mechanism of action. Sci. Rep. 2019, 9, 6387. [Google Scholar] [CrossRef] [PubMed]
- Campos, L.E.; Garibotto, F.; Angelina, E.; Kos, J.; Gonec, T.; Marvanova, P.; Vettorazzi, M.; Oravec, M.; Jendrzejewska, I.; Jampilek, J.; et al. Hydroxynaphthalenecarboxamides and substituted piperazinylpropandiols, two new series of BRAF inhibitors. A theoretical and experimental study. Bioorg. Chem. 2020, 103, 104145. [Google Scholar] [CrossRef] [PubMed]
- Capriotti, K.; Capriotti, J.A. Dimethyl sulfoxide. J. Clin. Aesthet. Dermatol. 2012, 5, 24–26. [Google Scholar] [PubMed]
- Bioscreen C–Mbio.ncsu.edu, User’s Manual. Yumpu.Com. Available online: https://www.yumpu.com/en/document/read/48827581/bioscreen-c-mbioncsuedu (accessed on 26 September 2023).
- De Marco, A.; De Candia, M.; Carotti, A.; Cellamare, S.; De Candia, E.; Altomare, C. Lipophilicity-related inhibition of blood platelet aggregation by nipecotic acid anilides. Eur. J. Pharm. Sci. 2004, 22, 153–164. [Google Scholar] [CrossRef] [PubMed]
- National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; M07; NCCLS: Wayne, PA, USA, 2018. [Google Scholar]
 | |||
|---|---|---|---|
| No. | R | MIC (µM) | |
| E. coli | S. typhimurium | ||
| 1 | H | >1000 | >1000 |
| 2 | 2-OCH3 | 900 | 1000 |
| 3 | 3-OCH3 | 200 | 200 |
| 4 | 2-OC2H5 | 300 | 300 |
| 5 | 3-OC2H5 | 400 | 200 |
| 6 | 4-OC2H5 | 600 | 600 |
| 7 | 2-OC3H7 | 500 | 200 |
| 8 | 3-OC3H7 | 300 | 200 |
| 9 | 4-OC3H7 | 500 | 200 |
| 10 | 2-OC4H9 | 400 | 200 |
| 11 | 3-OC4H9 | 300 | 200 |
| 12 | 4-OC4H9 | 400 | 300 |
| 13 | 2-OCH(CH3)2 | 400 | 200 |
| 14 | 3-OCH(CH3)2 | 300 | 200 |
| 15 | 4-OCH(CH3)2 | 400 | 300 |
| 16 | 2-OCH(CH3)CH2CH3 | 100 | 50 |
| 17 | 3-OCH(CH3)CH2CH3 | 400 | 300 |
| 18 | 4-OCH(CH3)CH2CH3 | 200 | 200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolaeva, V.; Gonec, T.; Kushkevych, I.; Jampilek, J. Effects of N-(Alkoxyphenyl)-1-hydroxynaphthalene-2-carboxamides on Intestinal Microbial Communities. Chem. Proc. 2023, 14, 45. https://doi.org/10.3390/ecsoc-27-16142
Nikolaeva V, Gonec T, Kushkevych I, Jampilek J. Effects of N-(Alkoxyphenyl)-1-hydroxynaphthalene-2-carboxamides on Intestinal Microbial Communities. Chemistry Proceedings. 2023; 14(1):45. https://doi.org/10.3390/ecsoc-27-16142
Chicago/Turabian StyleNikolaeva, Vanina, Tomas Gonec, Ivan Kushkevych, and Josef Jampilek. 2023. "Effects of N-(Alkoxyphenyl)-1-hydroxynaphthalene-2-carboxamides on Intestinal Microbial Communities" Chemistry Proceedings 14, no. 1: 45. https://doi.org/10.3390/ecsoc-27-16142
APA StyleNikolaeva, V., Gonec, T., Kushkevych, I., & Jampilek, J. (2023). Effects of N-(Alkoxyphenyl)-1-hydroxynaphthalene-2-carboxamides on Intestinal Microbial Communities. Chemistry Proceedings, 14(1), 45. https://doi.org/10.3390/ecsoc-27-16142








