Abstract
Five-membered saturated metallacarbocycles represent a large family of organometallic compounds, which are frequently postulated as reactive intermediates in catalysis or as precursors for the synthesis of a wide range of functionally substituted compounds, however, their NMR spectral data are incomplete and not systematized. Metallacarbocycles for Main III Group metals, which are spectroscopically characterized, are described in this article. Among these, of particular interest are 1-ethyl-3-substituted alumolanes, 3-spiro-substituted polycyclic alumolanes and nonbornen annelated alumolanes, which are supposedly formed by alkene cycloalumination with AlEt3 catalyzed by Cp2ZrCl2. Conformational analysis upon inversion of a five-membered ring for mono and polycyclic alumolanes is presented.
1. Introduction
Five-membered saturated metallacarbocycles for Main III Group metals, such as alumolanes, gallolanes and indolanes (tallolanes are not described in the literature) are frequently postulated as precursors for the synthesis of functionally substituted compounds. In laboratory practice, as a rule, metallacycles are not isolated in pure form due to their high sensitivity to moisture and atmospheric oxygen; therefore, their identification is carried out by the products of subsequent oxidation and deuterolysis. For example, the identification of 1-chloro-3-alkyl-indacyclopentanes [1], which were synthesized in the reaction of α-olefins with indium chloride and magnesium metal using i-Bu2AlH catalyzed by Cp2ZrCl2, was carried out exclusively on the basis of hydrolysis and deuterolysis products. Gallacyclopentanes, which are described by NMR spectroscopy, are not numerous and are represented in the review by three compounds 1–3 (Scheme 1). Gallacyclopentanes 1 and 2 are obtained by reacting gallium dichloride Et2N(CH2)3GaCl2 with Li(CH2)4Li or BrMg(CH2)4MgBr at −78 °C in diethyl ether [2]. Compound 1 is a white solid at room temperature (Tmelt = 88 °C, Tbp = 125 °C), while gallacyclopentane 2, structurally similar to it, is a viscous liquid (Tbp = 53 °C) under standard conditions. Metallacycle 3 has the form of white crystals at room temperature (Tmelt = 288–291 °C), which made it possible to identify it using X-ray diffraction analysis [3]. It was shown that the five-membered rings in the dimeric structure of compound 3 are in the C2-symmetric twist conformation. The signals of the cyclic methylene groups located in the α-position to the metal in metallacyclopentanes 1 and 2 in the 1H and 13C NMR spectra appear in the ranges of 0.3–0.9 ppm and 13.07–13.98 ppm, respectively. The signals of the cyclic β-methylene groups of gallacyclopentanes in the 1H and 13C NMR spectra were recorded in the regions of 1.55–2.65 and 30.28–34.48 ppm, respectively.
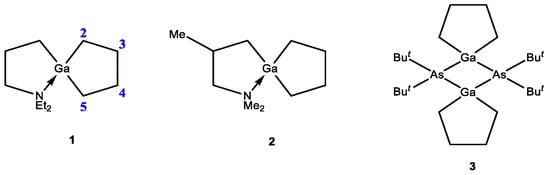
Scheme 1.
Structure of gallacyclopentanes.
Among the five-membered cyclic organic compounds containing an aluminum atom in their structure, 1-ethyl-3-substituted alumolanes are known, which are formed in the cycloalumination of α-olefins with AlEt3 catalyzed by Cp2ZrCl2 [4]. This method using alumolanes enabled the synthesis of a broad range of organic and heteroatom-containing compounds, including those that were difficult to synthesize before [5,6]. To date, the latest data indicate the possibility of using the reaction in the one-pot synthesis of phospholanes [7,8] and borolanes [9]. Since alumolanes are viscous homogeneous liquids, multinuclear 1H, 13C and 27Al NMR spectroscopy is the only reliable tool for structural studies. Unlike acyclic alkylalanes AlR3 (R = Me, Et, Pr, i-Bu), which have been structurally studied in detail [10,11,12,13], ring organoaluminum compounds (OACs) have received much less attention [14,15,16]. Recently, we carried out a systematic structural high-resolution multinuclear NMR study of alumolanes in polar solvents [17] and in non-polar media, where self-association processes take place similarly to acyclic alkylalanes.
2. Results and Discussion
A systematic analysis of a number of 1-ethyl-3-R-substituted alumolanes (R = n-Bu, n-Hex, n-Oct, i-Bu, Ph, Bn, SiMe3, SiEt3, cyclohex-2-en-1-yl) in polar (Et2O, THF, pyridine) and nonpolar (toluene, cyclohexane) solvents by multinuclear 1H, 13C, and 27Al NMR spectroscopy was carried out using two-dimensional (COSY, HSQC, HMBC) techniques (Scheme 2). We have assigned all the observed NMR signals of the atoms of cyclic OACs (for example, Figure 1). As follows from Figure 1, the signals of the carbon atoms located in the α-position to the metal atom are significantly broadened and appear in the high-field region of the spectrum: δC-2 = 13.6 ppm and δC-5 = 5.7 ppm. The two pairs of methylene protons corresponding to them are diastereotopic and appear in the two-dimensional COZY and HSQC spectra as geminal partners bound together in pairs. Analysis of the experimental 3JHH and 4JHH in the 1H NMR spectra (Figure 1), as well as theoretical conformational analysis, showed that the 3-substituted five-membered aluminum carbocycle in solution (Figure 2) is predominantly in the twist conformation with a pseudo-equatorial substituent in the third position.
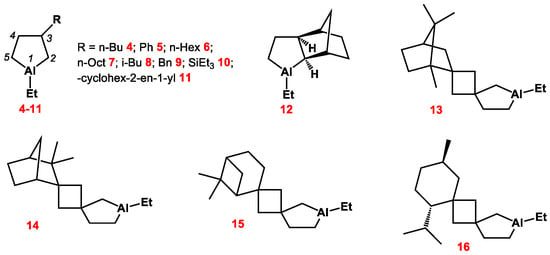
Scheme 2.
Structure of alumolanes.
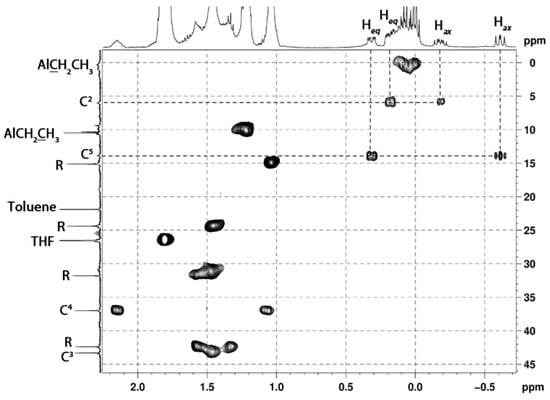
Figure 1.
Fragment of the HSQC spectrum of 1-ethyl-3-butylalumolane 4 in THF-d8.
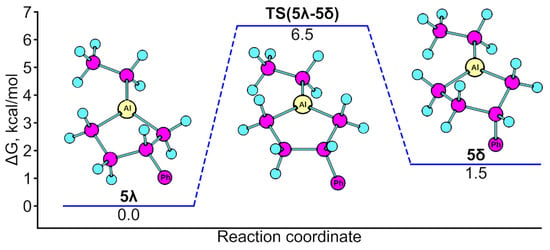
Figure 2.
Conformational analysis of 3(S)-alumolane 5.
The conformational rigidity of the five-membered aluminacarbocycle made it possible for cyclic OACs to determine the direct heteronuclear constants 1J(1H–13C) from the data of two-dimensional experiments without proton suppression for the first time. Thus, the values of the constants for the methylene fragments in the α-position to the aluminum atom vary within 108 ÷ 116 Hz. The lower value of the constants compared to those for ordinary Csp3–H carbon-hydrogen bonds (~120 Hz) is explained by the lower electron density on carbon atoms due to the influence of the metal atom. The stereospecificity of direct heteronuclear constants at C2H2, C5H2, where 1J(1Heq–13C) > 1J(1Hax–13C), was established, indicates the existence of stereoelectronic effects within the aluminacarbocycle.
The 27Al NMR signals of the spectra of 1-ethyl-3-R-substituted alumolanes in THF are observed in the region of δAl 178–185 ppm and in toluene δAl(4) = 146.3 ppm (W1/2 = 5.8 kHz), which indicates the presence of a four-coordinated aluminum atom in the molecular structure [18]. Despite the formation of diastereomers (Scheme 3) due to the complexation with Et2O, THF and Pyr solvent molecules at the metal atom, diastereomers were not observed even when the temperature was lowered to 200 K due to the rapid epimerization of the stereogenic center on Al.
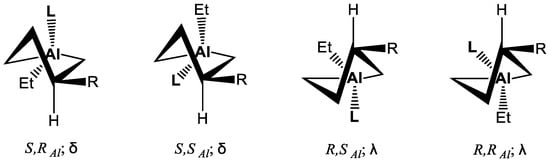
Scheme 3.
Diastereomers of solvated alumolane.
We calculated the thermodynamic parameters of complex formation reactions for each of the isomers using 1,3-diethylalumolane as an example. A comparative analysis of the calculated ∆G for the complexes of the model compound showed that the thermodynamic stability of the complexes decreases in the series: OAC∙Py > OAC∙THF > OAC∙Et2O. The most stable is the pyridine complex, in which the "lifetime" of the ligand on the metal atom is the longest. It is natural that only in the 13C NMR spectra of pyridine solutions of 1-ethyl-3-phenyl(butyl)alumolanes did we manage to detect the signals of two diastereomers.
A distinctive feature of the 1H and 13C NMR spectra of 1-ethyl-3-R-alumolanes in toluene is the presence of a large number of signals for each carbon atom of the molecule (Figure 3). As the temperature rises to 333 K, the spectrum simplifies due to the coalescence of a number of signals. We performed a quantum chemical evaluation of the thermodynamic and activation parameters of the alumolane dimerization reaction using 1,3-diethylalumolane as an example.
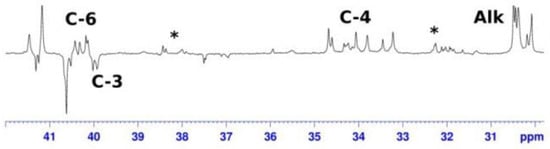
Figure 3.
13C NMR spectra (dept 135 of 1-ethyl-3-butylalumolane 4 in toluene (50% OAC): at T = 298 K (symbol * denotes minor carbalumination products).
As a result, possible isomer structures were calculated taking into account configurational and conformational isomerism, of which 20 dimeric forms can be stable at room temperature (∆G ≤ 0). The two most energetically favorable structures are shown in Figure 4.
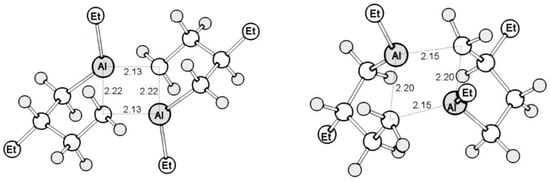
Figure 4.
Optimized structures of the two most energetically favorable dimers of 1,3-diethylalumolane: anti-SR-δλ (∆G = −34.3 kJ/mol) and SAlRAlSS-δδ (∆G = −30.1 kJ/mol).
As follows from the theoretical data that was obtained, stereoisomers of dimers should predominantly exist at room temperature, in which bridging bonds are formed with the participation of the metal–carbon bonds of the five-membered ring. The bonds formed in alumolane dimers, by analogy with alumols (according to PCA experiments [19]) and AlMe3 (according to new electron spectroscopy data [20]), should be interpreted in terms of multicenter binding.
We found that the theoretical and experimental data are consistent, so we also performed a conformational analysis for 3-spiro-substituted polycyclic alumolanes and nonbornen annelated alumolanes 12–16 [21]. The results are presented in Figure 5.
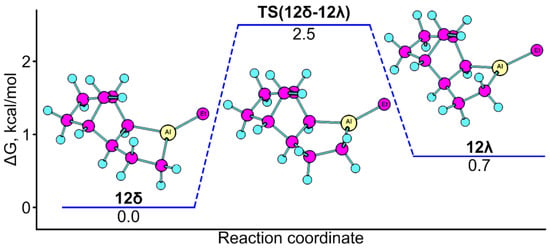
Figure 5.
Conformational analysis of alumolane 12.
As follows from Figure 5, conformations of alumolane exo-annelated with nonbornene were found on the potential energy surface of the molecule that differ in energy by 0.7 kcal/mol. Conformational dynamics are associated with the vibration (∆G≠ = 2.5 kcal/mol) of the methylene fragment (Al-CH2-) of the five-membered ring. The inversion of metal-lacycle in a spiro-linked compound requires significantly more energy; for example, the energy barrier for the conformational transition in alumolane 15 is 7.4 kcal/mol.
3. Conclusions
The NMR data of five-membered saturated metallacarbocycles for Main III Group metals have been summarized. Particular attention was paid to 1-ethyl-3-substituted alumolanes. The equilibrium mixtures of metallocyclic dimers are formed via the coordination of Al-C ring bonds in nonpolar solvents. The multicenter character of metal and C atom binding in alumolane dimers has been shown. In polar solvents, solvates of alumolanes with solvent molecules are formed. Aluminacarbocycle adopts a chiral twist conformation with a pseudo-equatorial 3-substituent position. Comparative conformational analysis upon the inversion of a five-membered ring for mono and polycyclic alumolanes was carried out.
Author Contributions
Conceptualization, T.V.T.; methodology, validation, and execution of chemistry experiments, manuscript preparation D.N.I. and T.V.T. All authors have read and agreed to the published version of the manuscript.
Funding
The work was conducted within the parameters of the approved plans for research projects at the IPC RAS State Registration No. FMRS-2022-0081.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the authors.
Acknowledgments
The studies were performed with the use of equipment from the Collective Usage Centre “Agidel” of Ufa Federal Research Centre of the Russian Academy of Science at the Institute Petrochemistry and Catalysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dzhemilev, U.M.; Ibragimov, A.G.; Ermilova, O.E.; Sultanov, R.M.; Khalilov, L.M.; Kunakova, R.V.; Sharipova, A.Z. Method of Preparing 1-Chloro-trans-3,4-dialkyl Substituted Indium. Cyclopentanes. Patent RU2164227C2, 20 January 1999. [Google Scholar]
- Schumann, H.; Just, O.; Seuß, T.D.; Görlitz, F.H.; Weimann, R. Intramolekular stabilisierte galla- und Indacyclohexane, -cyclopentane und -cyclopentene; Röntgenstrukturanalyse von (CH2)5Ga(CH2)3NM. J. Organomet. Chem. 1994, 466, 5–14. [Google Scholar] [CrossRef]
- Cowley, A.H.; Corbelin, S.; Jones, R.A.; Lagow, R.J.; Nail, J.W. Synthesis and structure of [(CH2)4Ga-μ-AstBu2]2. The first example of a gallacyclopentane. J. Organomet. Chem. 1994, 464, C1–C3. [Google Scholar] [CrossRef]
- Ibragimov, A.G.; Khafizova, L.O.; Satenov, K.G.; Khalilov, L.M.; Yakovleva, L.G.; Rusakov, S.V.; Dzhemilev, U.M. Synthesis and transformations of metallacycles. Russ. Chem. Bull. 1999, 48, 1574–1580. [Google Scholar] [CrossRef]
- Dzhemilev, U.M.; D’yakonov, V.A. Hydro-, carbo-, and cycloalumination of unsaturated compounds. In Modern Organoaluminum Reagents: Preparation, Structure, Reactivity and Use; Woodward, S., Dagorne, S., Eds.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2013; pp. 215–244. [Google Scholar]
- D’yakonov, V.A. Dzhemilev Reaction in Organic and Organometallic Synthesis; Nova Science Publishers, Inc.: New York, NY, USA, 2010. [Google Scholar]
- D’yakonov, V.A.; Makhamatkhanova, A.L.; Dilmukhametova, L.K.; Agliullina, R.A.; Tyumkina, T.V.; Dzhemilev, U.M. Catalytic cycloalumination for the synthesis of norbornane-annulated phospholanes. Organometallics 2015, 34, 221–228. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Makhamatkhanova, A.L.; Agliullina, R.A.; Dilmukhametova, L.K.; Tyumkina, T.V.; Dzhemilev, U.M. Aluminacyclopentanes in the synthesis of 3-substituted phospholanes and α,ω-bisphospholanes. Beilstein J. Org. Chem. 2016, 12, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Khafizova, L.O.; Khusainova, L.I.; Tyumkina, T.V.; Dzhemilev, U.M. One-pot synthesis of borolanes by reaction of aluminacyclopentanes with BF3·Et2O. Russ. J. Org. Chem. 2012, 48, 755–760. [Google Scholar] [CrossRef]
- Smith, M.B. The monomer−dimer equilibria of liquid aliminum alkyls: III. Trimethylaluminum: The monomer−dimer equilibria of liquid and gaseous trimethylaluminum and triethylaluminum. J. Organomet. Chem. 1972, 46, 31–49. [Google Scholar] [CrossRef]
- Yamamoto, O. The Low Temperature NMR Spectrum of Triethylaluminum. Bull. Chem. Soc. Jpn. 1964, 37, 1125–1128. [Google Scholar] [CrossRef]
- Yamamoto, O.; Hayamizu, K.; Yanagisawa, M. Bridge-terminal exchange of aluminum trialkyl dimers. J. Organomet. Chem. 1974, 73, 17–25. [Google Scholar] [CrossRef]
- Ramey, K.C.; O’Brien, J.F.; Hasegawa, I.; Borchert, A.E. Nuclear Magnetic Resonance Study of Aluminum Alkyls. J. Phys. Chem. 1965, 69, 3418–3423. [Google Scholar] [CrossRef]
- Dzhemilev, U.M.; Ibragimov, A.G.; Zolotarev, A.P.; Muslukhov, R.R.; Tolstikov, G.A. First preparative synthesis of alumocyclopentanes involving zirconium complexes. Russ. Chem. Bull. 1989, 38, 194–195. [Google Scholar] [CrossRef]
- Muslukhov, R.R.; Khalilov, L.M.; Zolotarev, A.P.; Morozov, A.B.; Ibragimov, A.G.; Dzhemilev, U.M.; Tolstikov, G.A. Synthesis and conversions of metallocycles. 8.13C NMR spectra of aluminocyclopentanes. Russ. Chem. Bull. 1992, 41, 1646–1651. [Google Scholar] [CrossRef]
- Dzhemilev, U.M.; Ibragimov, A.G. Regio- and stereoselective synthesis for a novel class of organoaluminium compounds—Substituted aluminacyclopentanes and aluminacyclopentenes assis. J. Organomet. Chem. 1994, 466, 1–4. [Google Scholar] [CrossRef]
- Tyumkina, T.V.; Islamov, D.N.; Parfenova, L.V.; Khalilov, L.M.; Dzhemilev, U.M. Structure and conformations of 2-substituted and 3-substituted alumolanes in polar solvents: A direct NMR observation. Magn. Reson. Chem. 2016, 54, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Benn, R.; Rufińska, A. High-Resolution Metal NMR Spectroscopy of Organometallic Compounds. Angew. Chem. Int. Ed. 1986, 25, 861–881. [Google Scholar] [CrossRef]
- Wasano, T.; Agou, T.; Sasamori, T.; Tokitoh, N. Synthesis, structure and reactivity of a 1-bromoalumole. Chem. Commun. 2014, 50, 8148–8150. [Google Scholar] [CrossRef] [PubMed]
- Stammler, H.-G.; Blomeyer, S.; Berger, R.J.F.; Mitzel, N.W. Trimethylaluminum: Bonding by Charge and Current Topology. Angew. Chem. Int. Ed. 2015, 54, 13816–13820. [Google Scholar] [CrossRef] [PubMed]
- Tyumkina, T.V.; Idrisova, S.M.; Nurislamova, R.R.; Tullyabaeva, L.I. Multinuclear 1H, 13C, 27Al and 31P NMR spectroscopy in the study of the structure of the 3-spiro-substituted polycyclic borolanes, alumolanes and phospholanes. In Proceedings of the International Conferences “Modern Development of Magnetic Resonance” and “Spin Physics, Spin Chemistry, and Spin Technology”, Kazan, Russia, 25–30 September 2023. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).