Synthesis of a Dysprosium(III) Complex with a Hexadentate Amine Ligand †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and General Methods
2.2. Synthesis
3. Results and Discussion
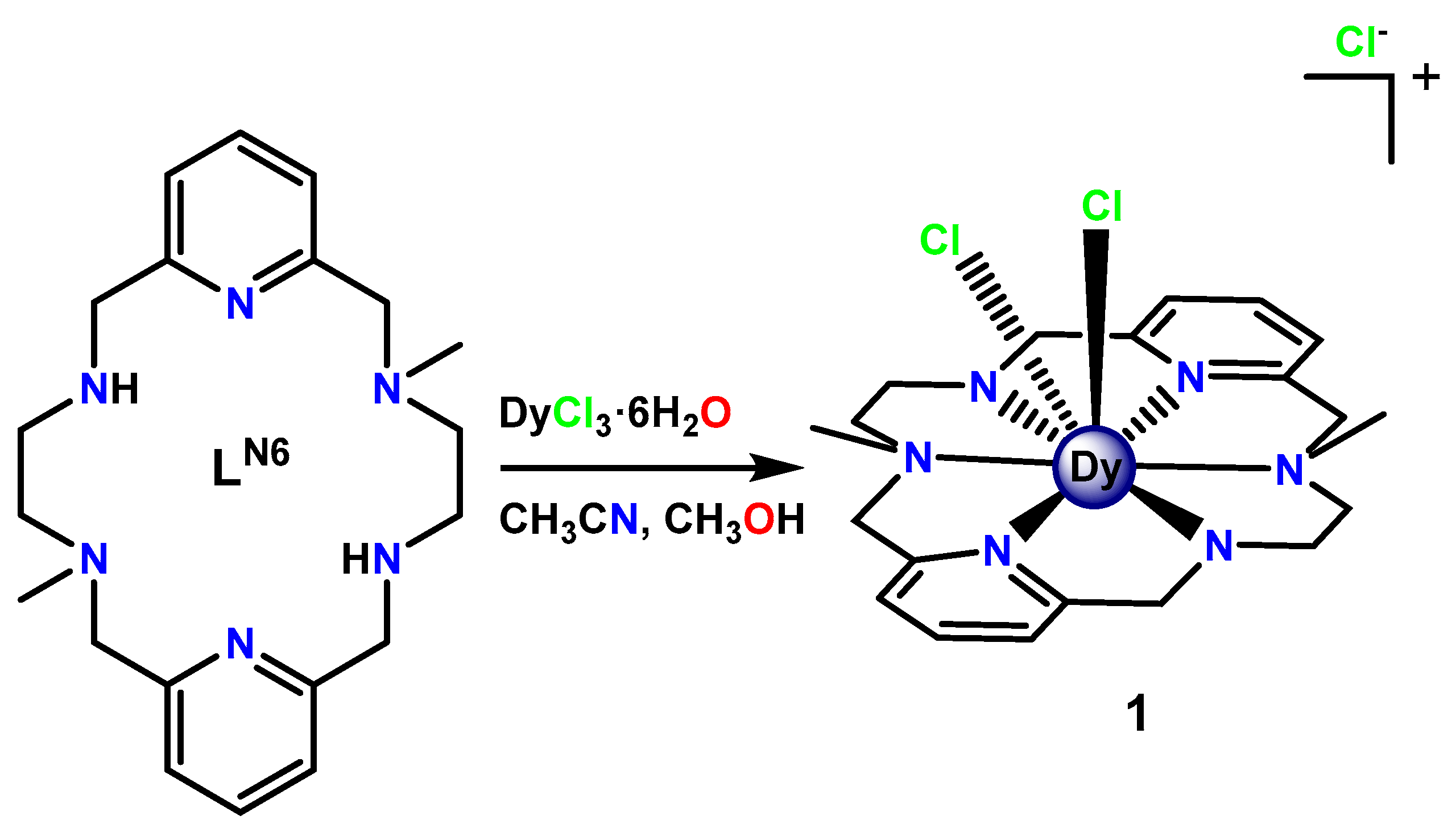
3.1. Synthesis
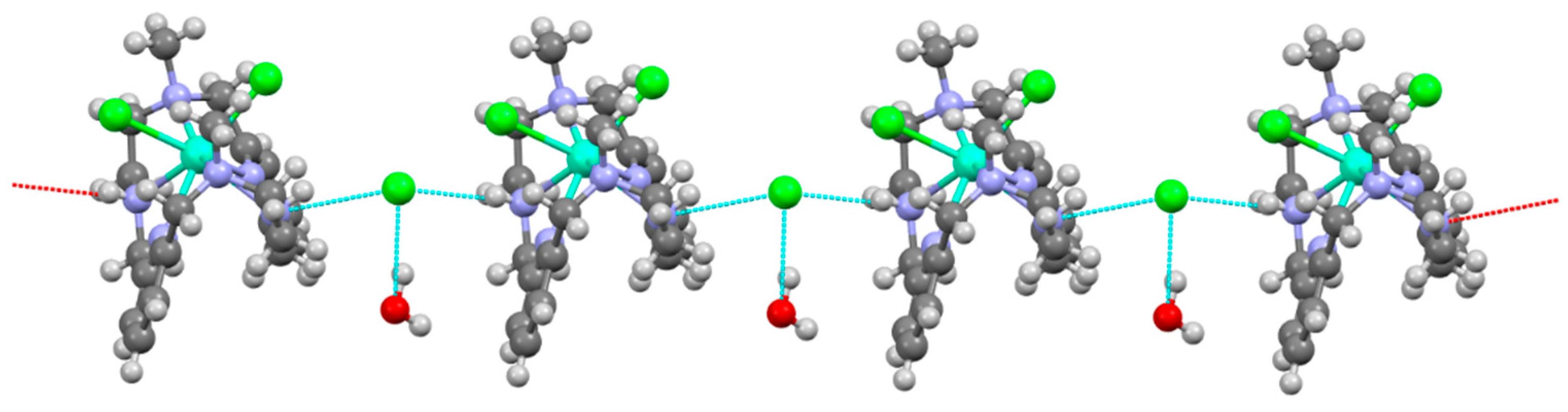
3.2. Single X-ray Diffraction Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mitewa, M.; Bontchev, P.R. Coordination chemistry of N6 macrocycles. Coord. Chem. Rev. 1994, 135–136, 129–163. [Google Scholar] [CrossRef]
- Rezaeivala, M.; Keypour, H. Schiff base and non-Schiff base macrocyclic ligands and complexes incorporating the pyridine moiety—The first 50 years. Coord. Chem. Rev. 2014, 280, 203–253. [Google Scholar] [CrossRef]
- Gavey, E.L.; Pilkington, M. Coordination complexes of 15-membered pentadentate aza, oxoaza and thiaaza Schiff base macrocycles “Old Complexes Offer New Attractions”. Coord. Chem. Rev. 2015, 296, 125–152. [Google Scholar] [CrossRef]
- Corredoira-Vázquez, J.; González-Barreira, C.; Oreiro-Martínez, P.; García-Deibe, A.M.; Sanmartín-Matalobos, J.; Fondo, M. Lanthanoid complexes of pentadentate and hexadentate N5 and N6 macrocycles: Synthesis and applications. J. Rare Earths 2023, in press. [Google Scholar] [CrossRef]
- Lamelas, R.; García, V.; Liñares, A.; Bastida, R.; Labisbal, E.; Fernández-Lodeiro, A.; Lodeiro, C.; Núñez, C.; Valencia, L. Novel trans-disubstituted hexaaza-macrocyclic ligands containing pyridine head units: Synthesis, disubstitution and colorimetric properties. Sens. Actuators B Chem. 2016, 225, 481–491. [Google Scholar] [CrossRef]
- Lamelas, R.; Bastida, R.; Labisbal, E.; Macías, A.; Pereira, T.; Pérez-Lourido, P.; Valencia, L.; Vila, J.M.; Núñez, C. A new series of lanthanide complexes with the trans-disubstituted Py2[18]aneN6 macrocyclic ligand: Synthesis, structures and properties. Polyhedron 2019, 160, 180–188. [Google Scholar] [CrossRef]
- Siemens Industrial Automation, Inc. SADABS: Area-Detector Absorption Correction; Siemens Industrial Automation, Inc.: Madison, WI, USA, 1996. [Google Scholar]
- Sheldrick, G.M. SHELXT-integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Llunell, M.; Casanova, D.; Cirera, J.; Bofill, J.M.; Alemany, P.; Alvarez, S.; Pinsky, M.; Avnir, D. SHAPE v1.1b; University of Barcelona: Barcelona, Spain, 2005. [Google Scholar]
- Ruiz-Martínez, A.; Casanova, D.; Alvarez, S. Polyhedral structures with an odd number of vertices: Nine-coordinate metal compounds. Chem. Eur. J. 2008, 14, 1291–1303. [Google Scholar] [CrossRef] [PubMed]
- Llunell, M.; Casanova, D.; Cirera, J.; Alemany, P.; Alvarez, S. SHAPE: Program for the Stereochemical Analysis of Molecular Fragments by Means of Continuous Shape Measures and Associated Tools; University of Barcelona: Barcelona, Spain, 2010. [Google Scholar]
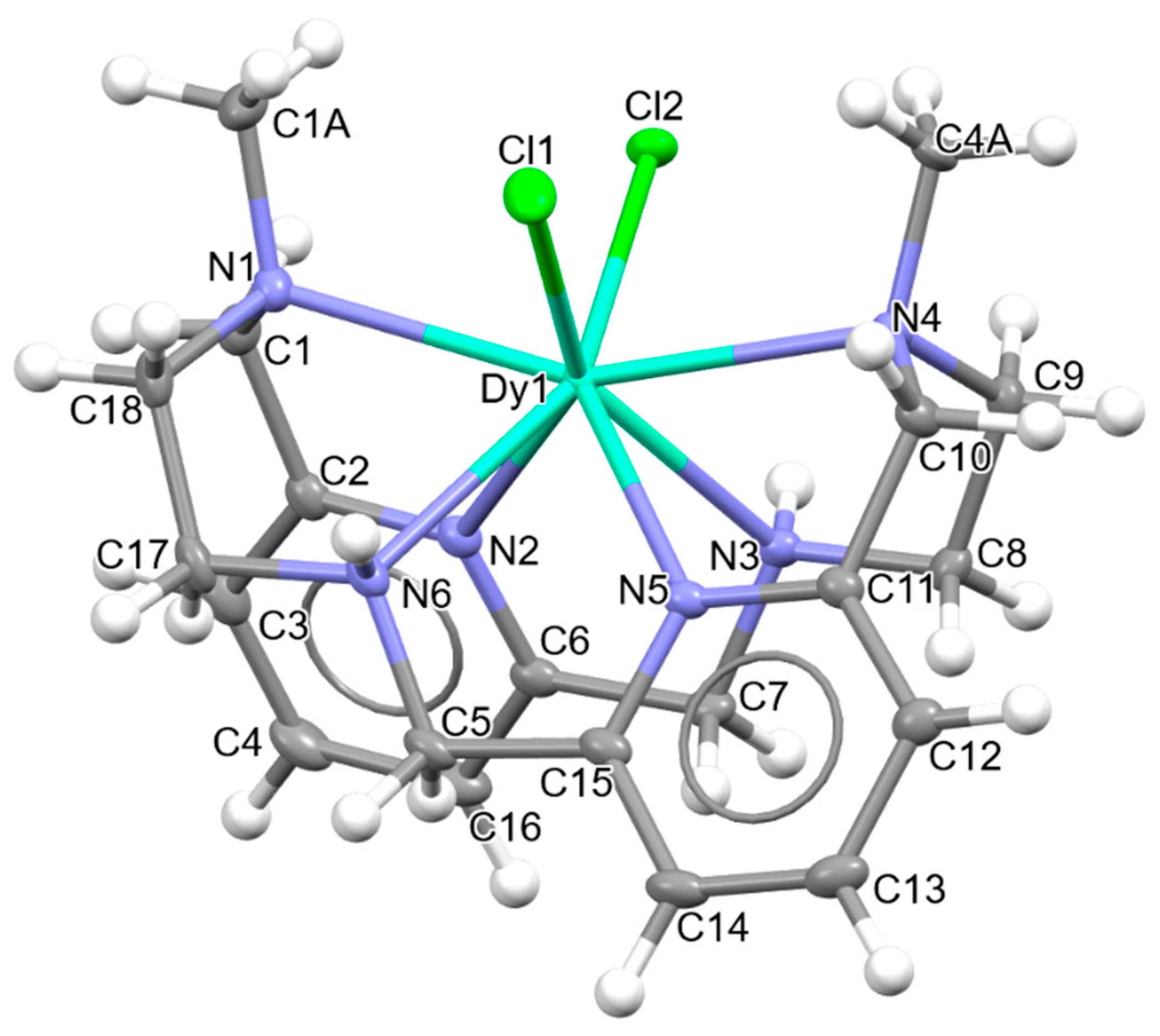
| Dy1-N1 | 2.6186(13) |
| Dy1-N2 | 2.5079(13) |
| Dy1-N3 | 2.5064(13) |
| Dy1-N4 | 2.6326(13) |
| Dy1-N5 | 2.4903(13) |
| Dy1-N6 | 2.5096(13) |
| Dy1-Cl1 | 2.6523(4) |
| Dy1-Cl2 | 2.6196(4) |
| N1-Dy1-N4 | 155.43(4) |
| N2-Dy1-N5 | 104.82(4) |
| N3-Dy1-N6 | 108.12(4) |
| Cl1-Dy1-Cl2 | 101.626(14) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Barreira, C.; Corredoira-Vázquez, J.; García-Deibe, A.M.; Fondo, M. Synthesis of a Dysprosium(III) Complex with a Hexadentate Amine Ligand. Chem. Proc. 2023, 14, 23. https://doi.org/10.3390/ecsoc-27-16143
González-Barreira C, Corredoira-Vázquez J, García-Deibe AM, Fondo M. Synthesis of a Dysprosium(III) Complex with a Hexadentate Amine Ligand. Chemistry Proceedings. 2023; 14(1):23. https://doi.org/10.3390/ecsoc-27-16143
Chicago/Turabian StyleGonzález-Barreira, Cristina, Julio Corredoira-Vázquez, Ana M. García-Deibe, and Matilde Fondo. 2023. "Synthesis of a Dysprosium(III) Complex with a Hexadentate Amine Ligand" Chemistry Proceedings 14, no. 1: 23. https://doi.org/10.3390/ecsoc-27-16143
APA StyleGonzález-Barreira, C., Corredoira-Vázquez, J., García-Deibe, A. M., & Fondo, M. (2023). Synthesis of a Dysprosium(III) Complex with a Hexadentate Amine Ligand. Chemistry Proceedings, 14(1), 23. https://doi.org/10.3390/ecsoc-27-16143









