Stability and Reactivity of Tocopherols: Theoretical Study †
Abstract
:1. Introduction
2. Methods
3. Results
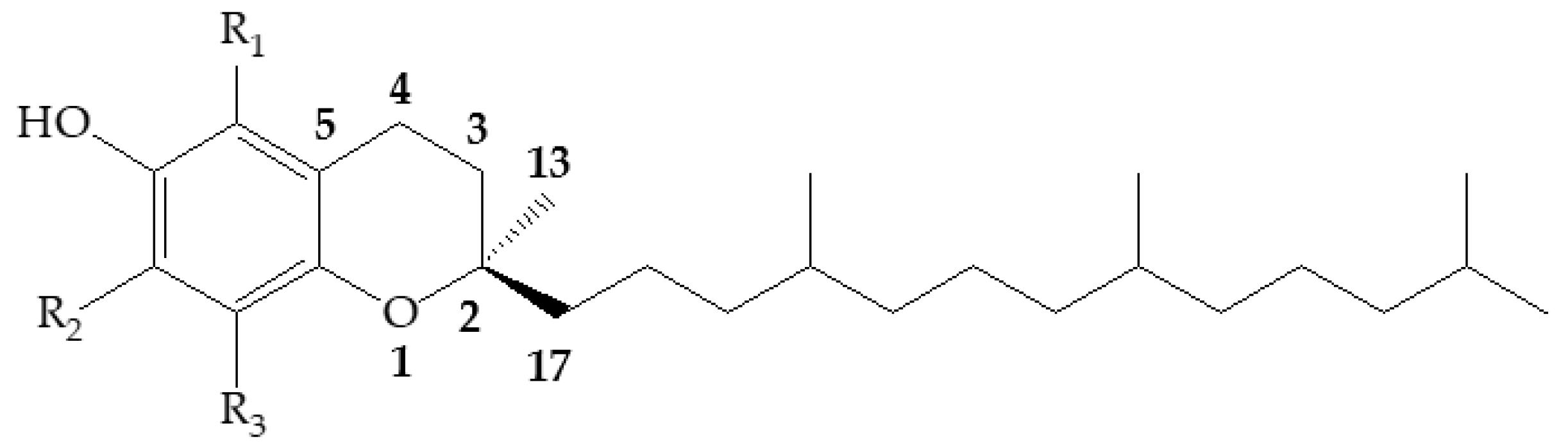
3.1. Structures
3.2. Dipolar Moment
3.3. Reactivity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Evans, H.M.; Bishop, K.S. On the Existence of a Hitherto Unrecognized Dietary Factor Essential for Reproduction. Science 1922, 56, 650–651. [Google Scholar] [CrossRef] [PubMed]
- Peh, H.Y.; Tan, W.S.D.; Liao, W.; Wong, W.S.F. Vitamin E Therapy beyond Cancer: Tocopherol versus Tocotrienol. Pharmacol. Ther. 2016, 162, 152–169. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q. Natural Forms of Vitamin E: Metabolism, Antioxidant, and Anti-Inflammatory Activities and Their Role in Disease Prevention and Therapy. Free. Radic. Biol. Med. 2014, 72, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Smit, H.A.; Grievink, L.; Tabak, C. Dietary Influences on Chronic Obstructive Lung Disease and Asthma: A Review of the Epidemiological Evidence. Proc. Nutr. Soc. 1999, 58, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Brzuskiewicz, L. Separation of Tocopherol and Tocotrienol Isomers Using Normal- and Reverse-Phase Liquid Chromatography. Anal. Biochem. 1989, 180, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Cuvelier, C.; Dotreppe, O.; Istasse, L. Chemistry, Nutritional Sources and Analyses of Vitamin E. Ann. De Med. Vet. 2003, 147, 315–324. [Google Scholar]
- Abidi, S.L. Chromatographic Analysis of Tocol-Derived Lipid Antioxidants. J. Chromatogr. A 2000, 881, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.-Y.; Htar, T.-T.; De Silva, L.; Tan, D.M.; Chuah, L.-H. Chromatographic Separation of Vitamin E Enantiomers. Molecules 2017, 22, 233. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Rainville, P. Chiral Separation of α-Tocopherol and α-Tocopheryl Acetate by Supercritical Fluid Chromatography for Accurate Vitamin E Nutrition Labeling. J. Food Compos. Anal. 2023, 117, 105135. [Google Scholar] [CrossRef]
- Yu, G.; Pawar, M. PEI-Loaded SiO2 as an Adsorbent for Separation of α-Tocopherol from Tocopherol Homologues. Monatshefte Für Chem. Chem. Mon. 2023, 154, 65–70. [Google Scholar] [CrossRef]
- Tsimidou, M.Z.; Mantzouridou, F.T.; Nenadis, N. Chapter Two—Minor Bioactive Lipids. In Advances in Food and Nutrition Research; Gallegos, C., Ruiz-Méndez, M.-V., Eds.; Academic Press: Cambridge, MA, USA, 2023; Volume 105, pp. 51–95. ISBN 1043-4526. [Google Scholar]
- Stewart, J.J.P. Optimization of Parameters for Semiempirical Methods II. Applications. J. Comput. Chem. 1989, 10, 221–264. [Google Scholar] [CrossRef]
- Glendening, E.D.; Reed, A.E.; Carpenter, J.E.; Weinhold, F. NBO, Version 3.1; Gaussian Inc.: Pittsburgh, PA, USA, 2003. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Hypercube Inc. Scientific Software Company, Incorporated in 1985. Available online: https://www.chemits.com/en/software/molecular-modeling/hyperchem/ (accessed on 14 October 2023).
- Fabijanić, I.; Jakobušić Brala, C.; Pilepić, V. The DFT Local Reactivity Descriptors of α-Tocopherol. J. Mol. Model. 2015, 21, 99. [Google Scholar] [CrossRef] [PubMed]


| Isoforms | Level | C(5)-C(4)-C(3)-C(2) 1 | C(4)-C(3)-C(2)-C(17) 1 | C(4)-C(3)-C(2)-C(13) 1 |
|---|---|---|---|---|
| α | B3Lyp/6-31G* | 43.2 | −171.7 | 61.0 |
| PM3 | 42.5 | −172.8 | 62.9 | |
| B3LYP/6-311++G** [16] | 43.1 | −171.7 | 61.0 | |
| B3LYP/6-311++G** [16] | −43.9 | −60.0 | 172.8 | |
| β | DFT | 44.1 | −171.3 | 61.3 |
| PM3 | 43.8 | −172.1 | 63.9 | |
| γ | DFT | 44.7 | −171.7 | 61.2 |
| PM3 | 44.0 | −172.9 | 63.8 | |
| δ | DFT | 45.8 | −171.9 | 61.0 |
| PM3 | 44.7 | −172.9 | 63.9 |
| System | α-Tocopherol | β-Tocopherol | γ-Tocopherol | δ-Tocopherol |
|---|---|---|---|---|
| DFT 1 | 0.80 | 0.99 | 1.02 | 1.19 |
| DFT 2 | 0.95 | 1.25 | 1.13 | 1.37 |
| PM3 | 0.68 | 0.95 | 0.97 | 0.98 |
| System | α-Tocopherol | β-Tocopherol | γ-Tocopherol | δ-Tocopherol |
|---|---|---|---|---|
| Gas | 0.1948 | 0.1969 | 0.1950 | 0.1928 |
| water | 0.1928 | 0.1947 | 0.1925 | 0.1946 |
| I | A | χ | µ | η | S | ω | ||
|---|---|---|---|---|---|---|---|---|
| α-Tocopherol | Gas state | 0.1818 | −0.0130 | 0.0844 | −0.0844 | 0.0974 | 10.2675 | 0.0366 |
| Aqueous solution | 0.1900 | −0.0028 | 0.0936 | −0.0936 | 0.0964 | 10.3751 | 0.0454 | |
| β-Tocopherol | Gas state | 0.1837 | −0.0132 | 0.0853 | −0.0853 | 0.0984 | 10.1580 | 0.0369 |
| Aqueous solution | 0.1916 | −0.0031 | 0.0943 | −0.0943 | 0.0973 | 10.2727 | 0.0457 | |
| γ-Tocopherol | Gas state | 0.1843 | −0.0107 | 0.0868 | −0.0868 | 0.0975 | 10.2543 | 0.0386 |
| Aqueous solution | 0.1926 | 0.0001 | 0.0963 | −0.0963 | 0.0963 | 10.3891 | 0.0482 | |
| δ-Tocopherol | Gas state | 0.1870 | −0.0059 | 0.0906 | −0.0906 | 0.0964 | 10.3724 | 0.0425 |
| Aqueous solution | 0.1946 | −0.0001 | 0.0973 | −0.0973 | 0.0973 | 10.2764 | 0.0486 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benbrahim, N.; Zeddour-Brahim, K.; Zizi, Z.; Bengharez, Z. Stability and Reactivity of Tocopherols: Theoretical Study. Chem. Proc. 2023, 14, 20. https://doi.org/10.3390/ecsoc-27-16043
Benbrahim N, Zeddour-Brahim K, Zizi Z, Bengharez Z. Stability and Reactivity of Tocopherols: Theoretical Study. Chemistry Proceedings. 2023; 14(1):20. https://doi.org/10.3390/ecsoc-27-16043
Chicago/Turabian StyleBenbrahim, Nasséra, Kawther Zeddour-Brahim, Zahia Zizi, and Zohra Bengharez. 2023. "Stability and Reactivity of Tocopherols: Theoretical Study" Chemistry Proceedings 14, no. 1: 20. https://doi.org/10.3390/ecsoc-27-16043
APA StyleBenbrahim, N., Zeddour-Brahim, K., Zizi, Z., & Bengharez, Z. (2023). Stability and Reactivity of Tocopherols: Theoretical Study. Chemistry Proceedings, 14(1), 20. https://doi.org/10.3390/ecsoc-27-16043






