An Optimised Method to Synthesise N5O2 Aminophenols †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and General Methods
2.2. Synthesis
3. Results and Discussion
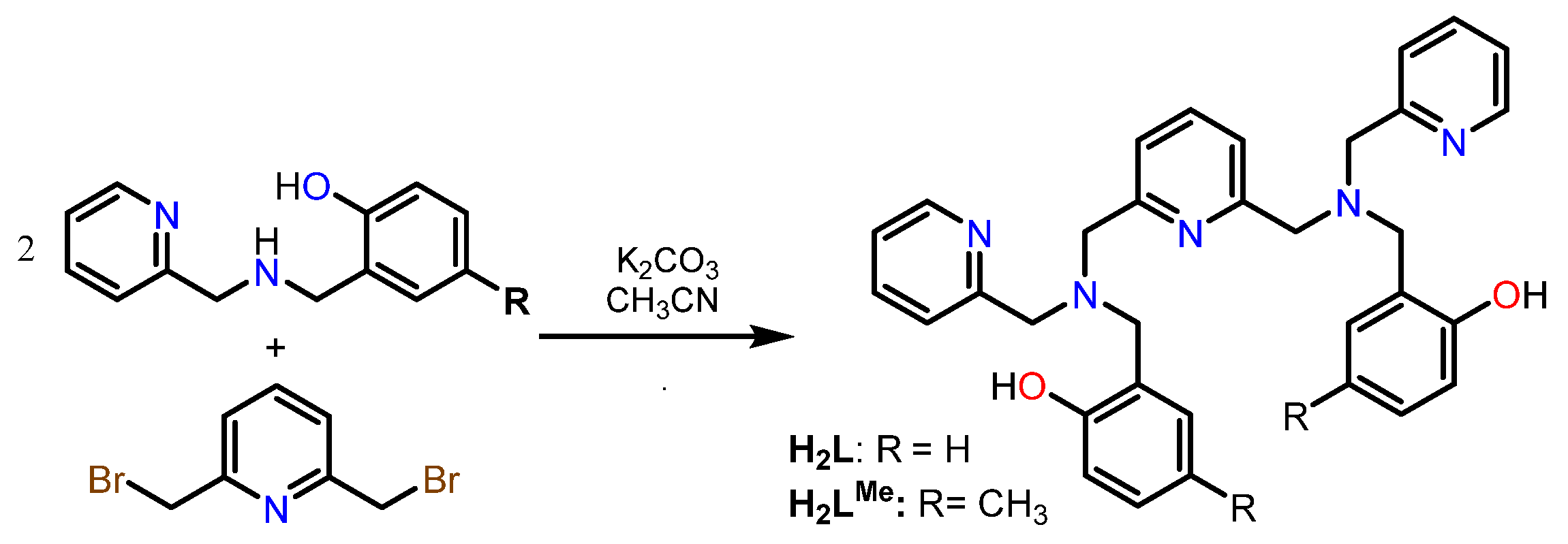
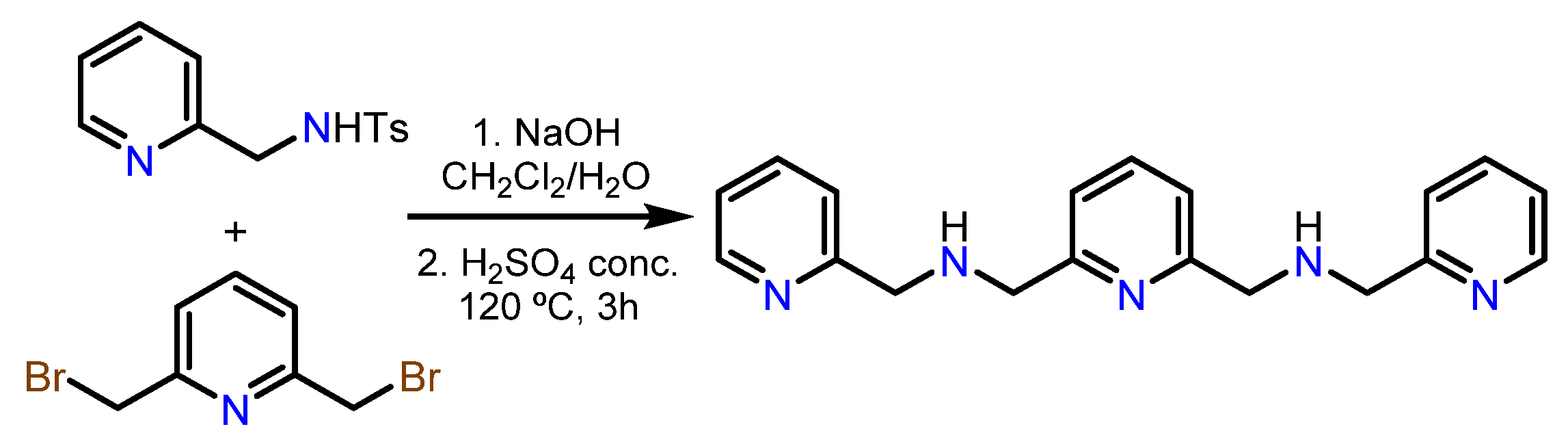
3.1. Synthesis of the Aminophenols
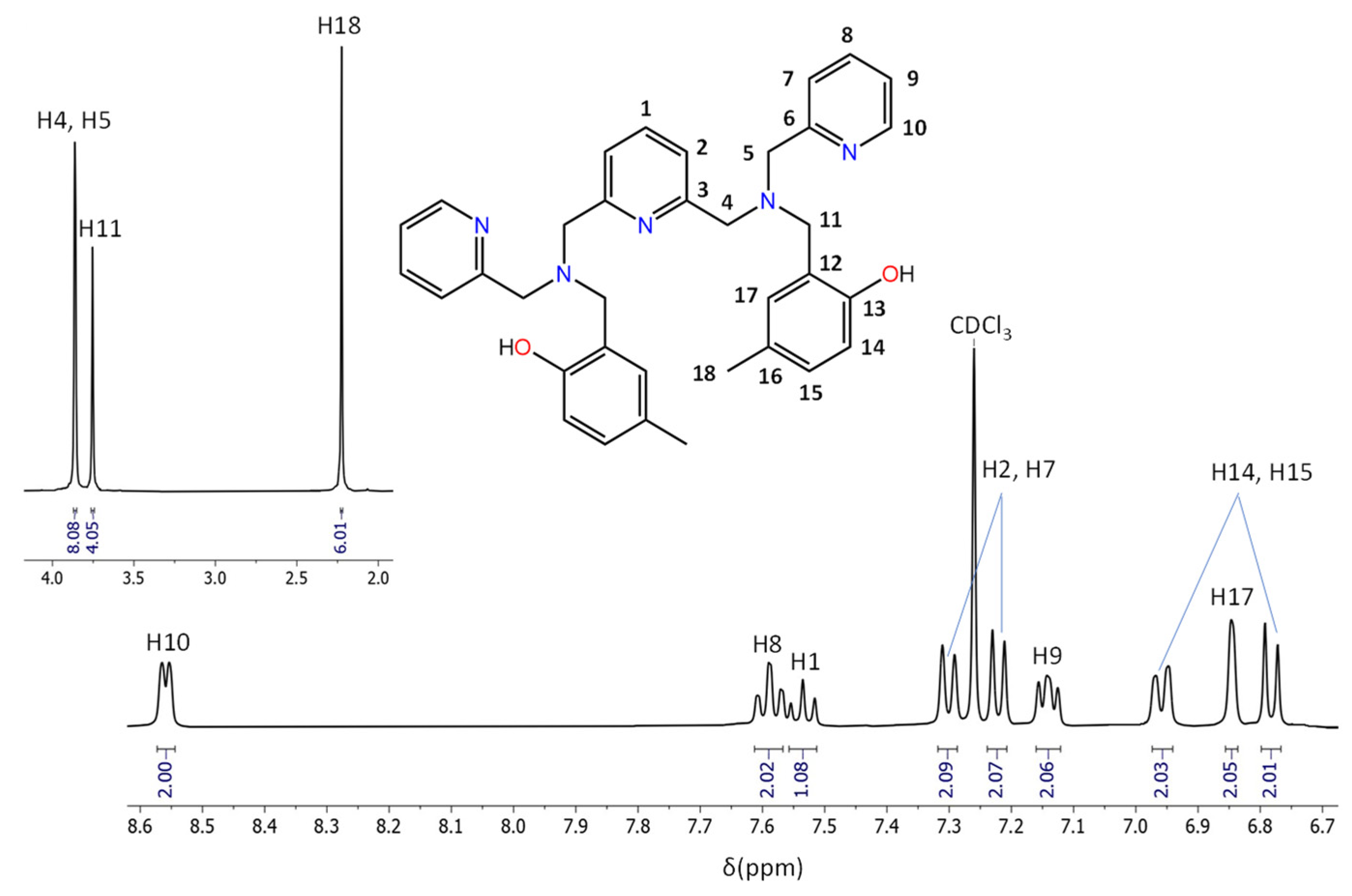
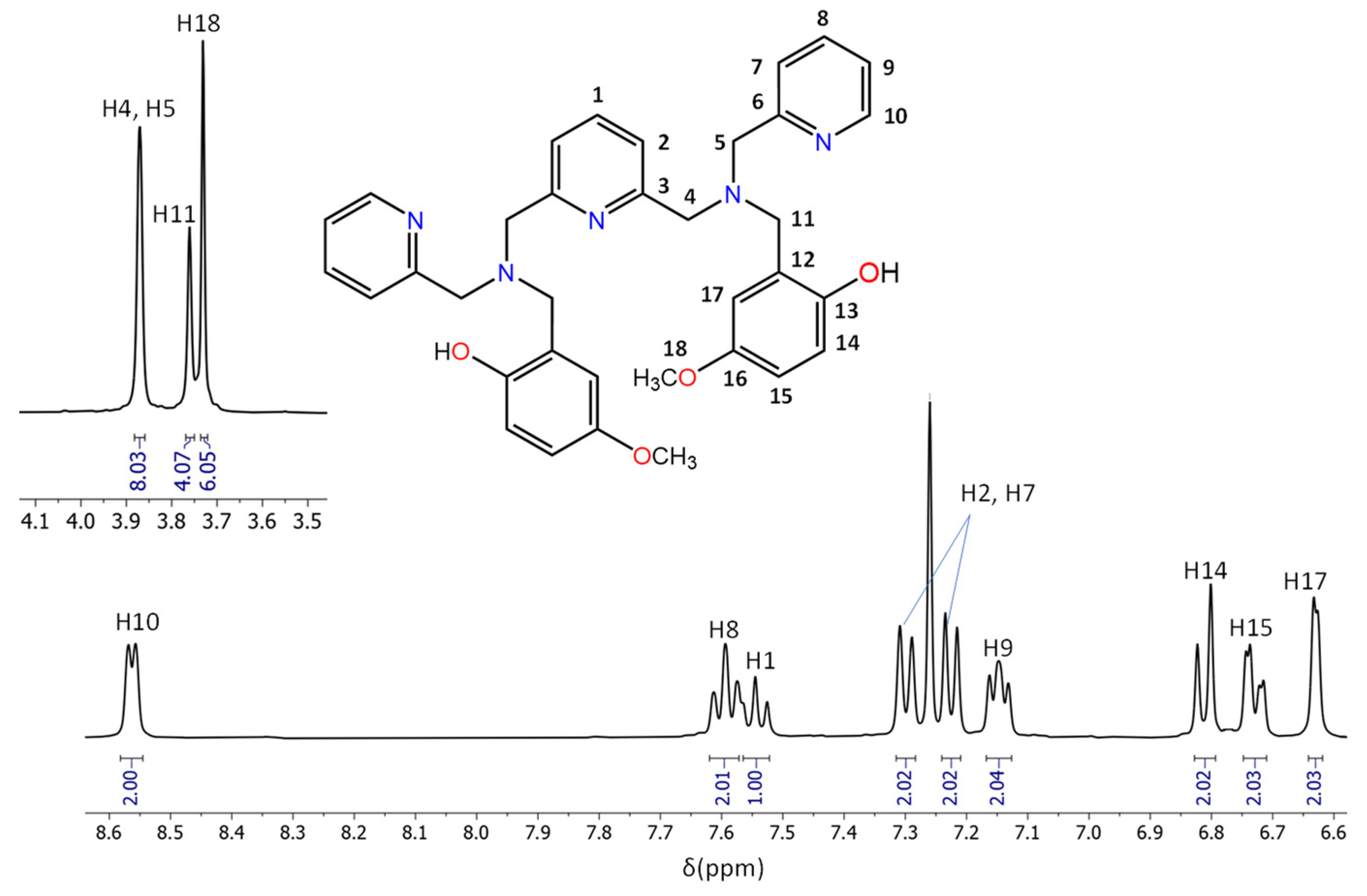
3.2. Characterisation of the Aminophenols
- The presence of two singlets in the region 3.5–4 ppm that integrate to 12 protons in total, indicating the existence of six CH2 groups and agreeing with the addition of the phenolic arms at the N5 precursor.
- The presence of nine signals in the aromatic region, which globally integrate to 17 protons, in agreement with the five aromatic rings, and, therefore, with the correct addition of the R-phenol to the N5 precursor.
- The presence of a singlet at 10 ppm (2H) and a second singlet at 2.3 ppm (6H) for H2LMe and at 3.73 for H2LOMe (6H), assigned to the hydroxyl and CH3 groups, respectively, also indicates the successful binding of the R-phenol to the precursor.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Çesme, M. 2-Aminophenol-based ligands and Cu(II) complexes: Synthesis, characterization, X-ray structure, thermal and electrochemical properties, and in vitro biological evaluation, ADMET study and molecular docking simulation. J. Mol. Struct. 2023, 1271, 134073. [Google Scholar] [CrossRef]
- Nakai, H.; Nonaka, K.; Goto, T.; Seo, J.; Matsumoto, T.; Ogo, S. A macrocyclic tetraamine bearing four phenol groups: A new class of heptadentate ligands to provide an oxygen-sensitive luminescent Tb(III) complex with an extendable phenol pendant arm. Dalton Trans. 2015, 44, 10923–10927. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lorraine, S.C.; Dolinar, B.S.; Hoover, J.M. Aerobic oxidation reactivity of well-defined cobalt(II) and cobalt(III) aminophenol complexes. Inorg. Chem. 2022, 61, 6008–6016. [Google Scholar] [CrossRef] [PubMed]
- Corredoira-Vázquez, J.; Oreiro-Martínez, P.; Nieto-Pastoriza, D.; García-Deibe, A.M.; Sanmartín-Matalobos, J.; Fondo, M. Dy4, Dy5 and Ho2 complexes o fan N3O2 aminophenol donor: A Dy3-µ3-peroxide single molecule magnet. Int. J. Mol. Sci. 2023, 24, 9061. [Google Scholar] [CrossRef] [PubMed]
- Rinehart, J.D.; Long, J.R. Exploiting single-ion anisotropy in the desing of f-elements in single-molecule magnets. Chem. Sci. 2011, 2, 2018–2085. [Google Scholar] [CrossRef]
- You, X.; Wei, Z.; Wang, H.; Li, D.; Liu, J.; Xu, B.; Liu, X. Synthesis of two copper clusters and their catalysis towards the oxidation of benzene into phenol. RSC Adv. 2014, 4, 61790–61798. [Google Scholar] [CrossRef]
- You, X.; Wei, Z.; Xu, B.; Liu, X. A heptadentate ligand possessing two phenol groups: Its diverse coordination chemistry and the catalytic behaviors of its transition complexes towards benzene oxidation. Polyhedron 2014, 81, 743–748. [Google Scholar] [CrossRef]
- Xu, S.-M.; An, Z.-W.; Zhang, W.; Zhang, Y.-Q.; Yao, M.-X. Ligand field and anion-driven structures and magnetic properties of dysprosium complexes. CrystEngComm 2021, 23, 2825–2834. [Google Scholar] [CrossRef]
- Meng, X.; Wang, M.; Gou, X.; Lan, W.; Jia, K.; Wang, Y.-X.; Zhang, Y.-Q.; Shi, W.; Cheng, P. Two C2v symmetry dysprosium(III) single-molecule magnets with effective energy barriers over 600 K. Inorg. Chem. Front. 2021, 8, 2349–2355. [Google Scholar] [CrossRef]
- Darbre, T.; Dubs, C.; Rusanov, E.; Stoeckli-Evans, H. Syntheses of Zinc Complexes with Multidentate Nitrogen Ligands: New Catalysts for Aldol Reactions. Eur. J. Inorg. Chem. 2002, 2002, 3284–3291. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oreiro-Martínez, P.; Corredoira-Vázquez, J.; Sanmartín-Matalobos, J.; Fondo, M. An Optimised Method to Synthesise N5O2 Aminophenols. Chem. Proc. 2023, 14, 17. https://doi.org/10.3390/ecsoc-27-16145
Oreiro-Martínez P, Corredoira-Vázquez J, Sanmartín-Matalobos J, Fondo M. An Optimised Method to Synthesise N5O2 Aminophenols. Chemistry Proceedings. 2023; 14(1):17. https://doi.org/10.3390/ecsoc-27-16145
Chicago/Turabian StyleOreiro-Martínez, Paula, Julio Corredoira-Vázquez, Jesús Sanmartín-Matalobos, and Matilde Fondo. 2023. "An Optimised Method to Synthesise N5O2 Aminophenols" Chemistry Proceedings 14, no. 1: 17. https://doi.org/10.3390/ecsoc-27-16145
APA StyleOreiro-Martínez, P., Corredoira-Vázquez, J., Sanmartín-Matalobos, J., & Fondo, M. (2023). An Optimised Method to Synthesise N5O2 Aminophenols. Chemistry Proceedings, 14(1), 17. https://doi.org/10.3390/ecsoc-27-16145