Abstract
Flame retardants play a crucial role in mitigating the hazards associated with fires by impeding their ignition and spread. However, conventional halogen-based flame retardants have encountered environmental and health concerns due to their persistence, bioaccumulation, and potential toxicity. In light of these concerns, the present study aimed to develop innovative compounds with potential applications as a flame retardant system that mitigates the drawbacks associated with halogen-based compounds. Several phosphoramidates were synthesized in a single step under mild conditions from the H-phosphonate dibenzo[1,3,2]dioxaphosphepine-6-oxide (BPPO), following a method based on the oxidation of the reactant in the presence of a suitable aliphatic or aromatic amine. The compounds were isolated with high purity, and the formulations were confirmed by multinuclear NMR spectroscopy.
1. Introduction
Since the initial report in 1972, the organophosphorus compound 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) and its derivatives have been known to be of noticeable industrial interest, being viable alternatives to halogenated flame retardants [1,2]. Being a H-phosphinate, DOPO shows two different tautomeric forms in equilibrium in solution, and it is thus able to behave both as a nucleophile and as an electrophile [3,4]. The reactivity of the P-H bond opens the possibility of formally replacing the hydrogen atom with several functional groups, allowing compounds to have specific properties but maintain flame-retardant activity both in the gas and condensed phases [5,6,7,8,9]. For instance, the P-H bond can be replaced with a P-C bond through reactions based on the nucleophilic attack on electron-poor carbon atoms [10,11,12,13,14,15], on the Michael addition [16,17,18,19,20], and on the Michaelis–Arbuzov rearrangement [21,22,23]. Phosphonamidates and phosphonates can be prepared from DOPO with the intermediate synthesis of 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-chloride (DOPO-Cl). Such a compound is generally obtained on the basis of the Atherton–Todd reaction using CCl4 as reactant [24,25,26,27,28,29], even if alternative chlorinating agents were considered, such as sulfuryl chloride, trichlorocyanuric acid, chlorine gas, and N-chlorosuccinimide [30,31,32,33,34].
Another cyclic phosphorus compound of growing interest in the field of flame reactants is the H-phosphonate dibenzo[1,3,2]dioxaphosphepine-6-oxide (BPPO) that can be isolated from a three-component reaction involving 2,2′-bisphenol, phosphorus trichloride, and water. The P-H bond revealed noticeable reactivity, and several organophosphorus BPPO derivatives can be prepared on the basis of phospha-Michael additions to unsaturated compounds [35,36,37]. It is worth noting that the different electron density on the phosphorus atom in BPPO with respect to DOPO alters the flame retardant behaviour since P-containing gases are preferentially released when low positive partial charges are present on the phosphorus atom. Hence, DOPO derivatives with low molecular weights mainly act in the gas phase, stopping the chain radical reaction, while the flame retardant action of BPPO-based compounds is more concentrated in the condensed phase, where the formation of a thermally stable char layer is promoted.
It is known that synergistic effects in the flame retardant behaviour can occur on mixing or reacting phosphorus- and nitrogen-based compounds [38,39,40,41,42,43]; therefore, the development of phosphoramidate (or amidophosphate) derivatives of BPPO appears as a promising approach to obtain flame retardants with tailored properties. Given our interest in cyclic organophosphorus compounds and phosphoramidates [44,45,46,47,48], some of us patented a straightforward approach for the preparation of BPPO derivatives with P-N bonds, working under mild conditions [49]. Herein, we report the synthesis and characterization of dibenzo[1,3,2]dioxaphosphepine-6-oxide phosphoramidates derived from butylamine, morpholine, 4-acetylpiperazine, aniline, and p-toluidine.
2. Materials and Methods
The reactants and solvents were Merck products and they were used as received. Dibenzo[1,3,2]dioxaphosphepine-6-oxide (BPPO) was synthesized according to a reported procedure [37]. In a 100 mL three-necked round-bottom flask equipped with condenser, magnetic stirring bar, dropping funnel, and nitrogen inlet, 2,2′-biphenol (25.75 g, 13.8 mmol) was dissolved in 50 mL of 1,4-dioxane and 2.5 mL of water and heated to reflux. A minimum flow of nitrogen was continuously passed through the solution. Phosphorus trichloride (12.1 mL, 13.8 mmol) was added within 3 h to the boiling reaction mixture. The generated HCl gas was absorbed in a trap filled with water. The reaction mixture was heated to reflux for an additional hour, then the solvent was removed under reduced pressure to obtain a viscous oil that became solid overnight. The product was triturated with 50 mL of diethyl ether, filtered, washed with fresh diethyl ether (10 mL), and dried under vacuum. Yield 75%.
Elemental analyses (C, H, N) were carried out using an Elementar Unicube microanalyzer. Melting points were registered using a FALC 360 D instrument equipped with a camera. Infrared (IR) spectra were registered using a Perkin-Elmer SpectrumOne spectrophotometer between 4000 and 450 cm−1 using KBr pellets. Mono- and bidimensional nuclear magnetic resonance (NMR) spectra were collected employing a Bruker Avance 400 instrument operating at 400.13 MHz of 1H resonance. 1H NMR spectra are referred to the partially non-deuterated fraction of the solvent, itself quoted with respect to tetramethylsilane. 31P{1H} chemical shifts are reported with respect to 85% H3PO4, with downfield shifts considered positive. 13C{1H} NMR spectra are referred to the solvent signal, quoted with respect to tetramethylsilane.
2.1. Synthesis of BPPO Phosphoramidate Derivatives
6-(butylamino)dibenzo[1,3,2]dioxaphosphepine-6-oxide (BPPO-NHButyl), 6-morpholinodibenzo[1,3,2]dioxaphosphepine-6-oxide (BPPO-Nmorph), 6-(4-acetylpiperazino)dibenzo[1,3,2]dioxaphosphepine-6-oxide (BPPO-NAcPz), 6-(phenylamino)dibenzo[1,3,2]dioxaphosphepine-6-oxide (BPPO-NHPh), and 6-(p-tolylamino)dibenzo[1,3,2]dioxaphosphepine-6-oxide (BPPO-NHTol) were all synthesized following the same general method. In a typical preparation, BPPO (1.15 g, 5.00 mmol) and 16.5 mmol of the proper amine (butylamine, 1.6 mL; morpholine, 1.4 mL; 1-acetylpiperazine, 2.13 g; aniline, 1.51 g; p-toluidine, 1.77 g) were dissolved in 30 mL of dichloromethane, then I2 (1.26 g, 5.00 mmol) was slowly added. The resulting reaction mixture was kept under vigorous stirring at room temperature for three hours. The solid by-product that separated with all the amines with the exception of butylamine was removed by filtration and washed with dichloromethane; then the organic solution was washed with brine (3 × 100 mL), dried with anhydrous Na2SO4 and filtered. The solvent was removed by evaporation under reduced pressure. In the case of the morpholine derivative, the raw product was purified by crystallization with cold ethanol (10 mL), followed by filtration. In the other cases, diethyl ether (10 mL) was added, and the solid formed was collected by filtration and washed with diethyl ether. All the products were finally dried under vacuum. Further product was collected from the diethyl ether solution in the case of the butylamine derivative after keeping the solution at −20 °C overnight. Yields: 30% (BPPO-NHButyl); 54% (BPPO-Nmorph); 12% (BPPO-NAcPZ); 94% (BPPO-NHPh); 60% (BPPO-NHTol).
2.1.1. Characterization of BPPO-NHbutyl
Anal. calcd for C16H18NO3P (303.29 g mol−1,%): C, 63.36; H, 5.98; N, 4.62. Found (%): C, 63.10; H, 6.00; N, 4.59. M.p. (°C): 101. IR (KBr, cm−1): 3355 νNH, 1243 νP=O. 1H NMR (CDCl3, 298 K): δ 7.53 (dd, 2H, JHH = 7.6 Hz, JHH = 1.8 Hz, arom), 7.42 (t, 2H, JHH = 7.6 Hz, arom), 7.33 (t, 2H, JHH = 7.6 Hz, arom), 7.29 (d, 2H, JHH = 7.6 Hz, arom), 3.05–2.90 (m, 3H, NH+CH2), 1.48 (m, 2H, CH2), 1.31 (m, 2H, CH2), 0.88 (t, 3H, JHH = 7.3 Hz, CH3). 31P{1H} NMR (CDCl3, 298 K): δ 13.36 (s). 13C{1H} NMR (CDCl3, 298 K): 148.12 (d, JPC = 9.4 Hz, arom-Cipso), 129.90 (d, JPC = 1.2 Hz, arom-CH), 129.78 (d, JPC = 1.2 Hz, arom-CH), 128.59 (d, JPC = 1.6 Hz, arom-Cipso), 126.50 (d, JPC = 1.8 Hz, arom-CH), 121.67 (d, JPC = 4.2 Hz, arom-CH), 41.92 (s, CH2), 34.03 (d, JPC = 5.5 Hz, CH2), 19.58 (s, CH2), 13.63 (s, CH3).
2.1.2. Characterization of BPPO-Nmorph
Anal. calcd for C16H16NO4P (317.28 g mol−1,%): C, 60.57; H, 5.08; N, 4.41. Found (%): C, 60.65; H, 5.10; N, 4.39. M.p. (°C): 171. IR (KBr, cm−1): 1251 νP=O. 1H NMR (CDCl3, 298 K): δ 7.53 (dd, 2H, JHH = 7.7 Hz, JHH = 1.7 Hz, arom), 7.44 (tdd, 2H, JHH = 7.7 Hz, JHH = 1.7 Hz, JPH = 0.9 Hz, arom), 7.35 (tt, 2H, JHH = 7.7 Hz, JHH = JPH = 1.2 Hz, arom), 7.32 (dt, JHH = 7.7 Hz, JHH = 1.2 Hz), 3.62 (m, 4H, O-CH2), 3.16 (m, 4H, N-CH2). 31P{1H} NMR (CDCl3, 298 K): δ 10.17 (s). 13C{1H} NMR (CDCl3, 298 K): δ 148.31 (d, JPC = 9.6 Hz, arom-Cipso), 130.04 (d, JPC = 1.2 Hz, arom-CH), 139.97 (d, JPC = 1.2 Hz, arom-CH), 128.23 (d, JPC = 1.4 Hz, arom-Cipso), 126.28 (d, JPC = 1.8 Hz, arom-CH), 121.70 (d, JPC = 4.4 Hz, arom-CH), 66.98 (d, JPC = 5.0 Hz, O-C), 45.47 (d, JPC = 1.0 Hz, N-C).
2.1.3. Characterization of BPPO-NAcPz
Anal. calcd for C18H19N2O4P (358.33 g mol−1,%): C, 60.33; H, 5.34; N, 7.82. Found (%): C, 60.09; H, 5.37; N, 7.78. M.p. (°C): 141. IR (KBr, cm−1): 1646 νC=O, 1251 νP=O. 1H NMR (CDCl3, 298 K): δ 7.55 (dd, 2H, JHH = 7.8 Hz, JHH = 1.2 Hz, arom), 7.44 (t, 2H, JHH = 7.4 Hz, arom), 7.36 (t, 2H, JHH = 7.4 Hz, arom), 7.30 (d, 2H, JHH = 7.8 Hz, arom), 3.53 (s, br, 2H, N-CH2), 3.44 (s, br, 2H, N-CH2), 3.22 (s, br, 2H, N-CH2), 3.09 (s, br, 2H, N-CH2), 2.08 (s, 3H, CH3). 31P{1H} NMR (CDCl3, 298 K): δ 9.54 (s). 13C{1H} NMR (CDCl3, 298 K): δ 169.17 (s, C=O), 148.16 (d, JPC = 9.6 Hz, arom-Cipso), 130.12 (d, JPC = 0.9 Hz, arom-CH), 130.05 (d, JPC = 1.0 Hz, arom-CH), 128.15 (d, JPC = 1.5 Hz, arom-CH), 126.42 (d, JPC = 1.8 Hz, arom-CH), 121.59 (d, JPC = 4.3 Hz, arom-CH), 46.73 (s, N-C), 45.35 (s, N-C), 41.66 (s, N-C), 21.30 (s, CH3).
2.1.4. Characterization of BPPO-NHPh
Anal. calcd for C18H14NO3P (323.28 g mol−1,%): C, 66.87; H, 4.36; N, 4.33. Found (%): C, 66.60; H, 4.38; N, 4.35. M.p. (°C): 155. IR (KBr, cm−1): 3378 νNH, 1197 νP=O. 1H NMR (CDCl3, 298 K): δ 7.55 (dd, 2H, JHH = 7.4 Hz, JHH = 2.1 Hz, arom), 7.42–7.33 (m, 4H, arom), 7.24–7.16 (m, 4H, arom), 7.07 (d, 2H, JHH = 7.9 Hz, arom), 7.01 (t, 1H, JHH = 7.4 Hz, arom), 5.44 (d, 1H, JPH = 8.0 Hz, NH). 31P{1H} NMR (CDCl3, 298 K): δ 6.28 (s). 13C{1H} NMR (CDCl3, 298 K): δ 147.84 (d, JPC = 9.3 Hz, arom-Cipso), 137.98 (d, JPC = 2.2 Hz, arom-Cipso), 130.12 (d, JPC = 1.4 Hz, arom-CH), 129.92 (d, JPC = 1.4 Hz, arom-CH), 129.33 (s, arom-CH), 128.41 (d, JPC = 1.7 Hz, arom-Cipso), 126.50 (d, JPC = 1.9 Hz, arom-CH), 123.20 (s, arom-CH), 121.80 (d, JPC = 4.4 Hz, arom-CH), 119.56 (d, JPC = 6.6 Hz, arom-CH).
2.1.5. Characterization of BPPO-NHtol
Anal. calcd for C19H16NO3P (337.31 g mol−1,%): C, 67.65; H, 4.78; N, 4.15. Found (%): C, 67.40; H, 4.80; N, 4.12. M.p. (°C): 189. IR (KBr, cm−1): 3379 νNH, 1198 νP=O. 1H NMR (CDCl3, 298 K): δ 7.54 (dd, 2H, JHH = 7.1 Hz, JHH = 1.8 Hz, arom), 7.42–7.31 (m, 4H, arom), 7.23 (d, 2H, JHH = 7.4 Hz, arom), 7.04–6.95 (m, 4H, arom), 5.45 (d, 1H, JPH = 7.6 Hz, NH), 2.27 (s, 3H, CH3). 31P{1H} NMR (CDCl3, 298 K): δ 6.16 (s). 13C{1H} NMR (CDCl3, 298 K): δ 147.95 (d, JPC = 9.5 Hz, arom-Cipso), 135.34 (d, JPC = 1.9 Hz, arom-Cipso), 132.74 (s, arom-Cipso), 130.05 (d, JPC = 0.8 Hz, arom-CH), 129.86 (d, JPC = 0.9 Hz, arom-CH), 129.78 (s, arom-CH), 128.46 (d, JPC = 1.5 Hz, arom-Cipso), 126.38 (d, JPC = 1.7 Hz, arom-CH), 121.83 (d, JPC = 4.4 Hz, arom-CH), 119.85 (d, JPC = 6.4 Hz, arom-CH), 20.66 (s, CH3).
3. Results and Discussion
According to the recently published patent [49], the conversion of BPPO is related phosphoramidates can be carried out in a single step by reacting the precursor with I2 in the presence of a suitable aliphatic or aromatic amine (amH), as depicted in Scheme 1. The compounds BPPO-NHButyl, BPPO-Nmorph, BPPO-NAcPz, BPPO-NHPh, and BPPO-NHTol were isolated with yields comprised between 12% and 94% and had a high degree of purity. The low yields obtained in some cases are mainly attributable to work-up issues. With the exception of butylammonium iodide, the by-product [amH2]I was always recovered by filtration from the reaction mixture. It is worth noting that an alternative synthetic approach for the preparation of BPPO-Nmorph is already present in the literature [50].
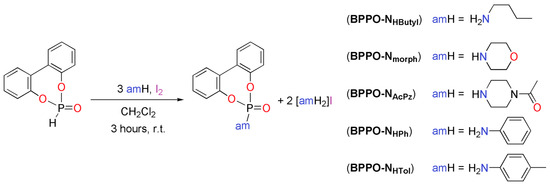
Scheme 1.
Synthesis of phosphoramidates from BPPO.
The 1H NMR spectra of all the compounds showed the disappearance of the P-H resonance of BPPO, while the four multiplets due to the equivalent aromatic rings of the biphenyl moiety were maintained. Besides the aromatic resonances, the 1H NMR spectrum of BPPO-NHButyl in CDCl3 shows the superposition of multiplets between 3.05 and 2.90 ppm assigned to the N-bonded hydrogen atom and the CH2 fragment. The other aliphatic resonances fall at 1.48, 1.31, and 0.88 ppm. The 13C{1H} NMR signals of the butyl chain fall in the 42–13 ppm range, and the one at 34.03 ppm exhibits a coupling constant of 5.5 Hz with the 31P nucleus, in agreement with the formation of the P-N bond. The presence of P-bonded morpholine in BPPO-Nmorph was highlighted by two multiplets at 3.62 and 3.16 ppm, correlated to resonances in the 13C{1H} NMR spectrum at 66.98 and 45.47 ppm. The 13C{1H} NMR signals are doublets thanks to the coupling with 31P. In the 1H NMR spectrum of the comparable BPPO-NAcPz compound, the four CH2 fragments of the piperazine heterocycle are all non-equivalent because of the different substituents at the nitrogen atoms and because of the lack of free rotation of the acetyl group around the N-C bond, that removes the equivalence of the two halves of the piperazine ring. The CH2 1H NMR chemical shift values are 3.53, 3.44, 3.22, and 3.09 ppm, correlated to 13C{1H} NMR resonances in the 47–41 ppm range. The acetyl substituent resonates at 2.08 ppm in the 1H NMR spectrum and at 169.17 and 21.30 ppm in the 13C {1H} NMR spectrum. The NMR spectra of BPPO-NHPh and BPPO-NHTol are similar, with obvious differences related to the presence in the second case of the methyl substituent. The NH resonance is clearly observable in the 1H NMR spectrum at about 5.45 ppm, with JPH coupling constant close to 8 Hz. The 1H NMR spectra are shown in Figure 1. A single sharp resonance was observed in all the 31P{1H} NMR spectra between 13.5 and 9.5 ppm for the derivatives of aliphatic amines and around 6.2 ppm for BPPO-NHPh and BPPO-NHTol (Figure 2).
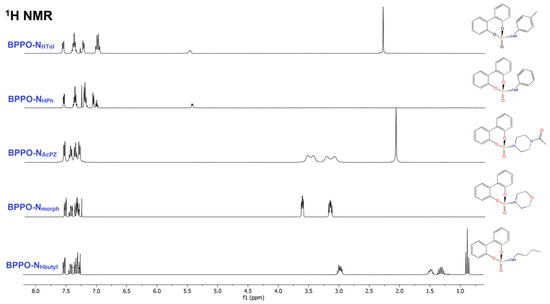
Figure 1.
1H NMR spectra of the phoshoramidate derivatives (CDCl3, 298 K).
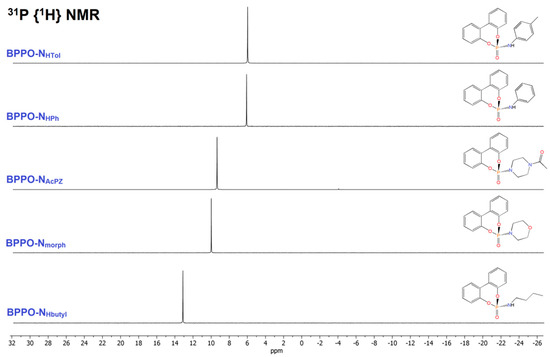
Figure 2.
31P{1H} NMR spectra of the phoshoramidate derivatives (CDCl3, 298 K).
The IR spectra show, in all the cases, the P=O stretching around 1200 cm−1. In some cases, further diagnostic signals were detected, such as the νNH stretching above 3300 cm−1 for BPPO-NHbutyl, BPPO-NHPh, and BPPO-NHTol, or the νCO stretching band at 1646 cm−1 for BPPO-NAcPz.
To conclude, in this article, we reported the straightforward synthesis of five phosphoramidates starting from BPPO, operating under mild conditions and avoiding the use of aggressive reactants. Most of the compounds described here are reported for the first time, and they are currently under investigation as flame retardants in combination with various plastics.
4. Patents
The data provided in this work were obtained on the basis of the 2023 patent WO2023094526A1, entitled “Preparation process of P(=O)-heteroatom derivatives of dibenzooxaphosphacycles”, presented by our research group.
Author Contributions
Conceptualization, M.B. and L.A.; methodology, M.B.; validation, M.B., G.M. and L.A.; formal analysis, M.B. and G.M.; investigation, G.M. and M.B.; resources, L.A.; data curation, M.B. and G.M.; writing—original draft preparation, M.B.; writing—review and editing, G.M. and L.A.; visualization, M.B. and G.M.; project administration, L.A.; funding acquisition, L.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon request due to restrictions related to the patent above reported.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Saito, T. Cyclic Organophosphorus Compounds and Process for Making Same. US3702878A, 14 November 1972. [Google Scholar]
- Pack, S. A Review of Non-halogen Flame Retardants in Epoxy-Based Composites and Nanocomposites: Flame Retardancy and Rheological Properties. In Flame Retardants; Visakh, P.M., Arao, Y., Eds.; Springer: Heidelberg, Germany, 2015; pp. 115–130. [Google Scholar]
- Stawinski, J.; Kraszewski, A. How To Get the Most Out of Two Phosphorus Chemistries. Studies on H-Phosphonates. Acc. Chem. Res. 2002, 35, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Montchamp, J.-L. Phosphinate Chemistry in the 21st Century: A Viable Alternative to the Use of Phosphorus Trichloride in Organophosphorus Synthesis. Acc. Chem. Res. 2014, 47, 77–87. [Google Scholar] [CrossRef]
- Artner, J.; Ciesielski, M.; Walter, O.; Döring, M.; Perez, R.M.; Sandler, J.K.W.; Altstädt, V.; Schartel, B. A Novel DOPO-Based Diamine as Hardener and Flame Retardant for Epoxy Resin Systems. Macromol. Mater. Eng. 2008, 293, 503–514. [Google Scholar] [CrossRef]
- Rakotomalala, M.; Wagner, S.; Döring, M. Recent Developments in Halogen Free Flame Retardants for Epoxy Resins for Electrical and Electronic Applications. Materials 2010, 3, 4300–4327. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-L.; Liu, L.-C.; Chen, C.-M.; Lin, J.-S. Syntheses and flame retarding properties of DOPO polymers, melamine polymers, and DOPO-melamine copolymers. Polym. Adv. Technol. 2014, 25, 36–40. [Google Scholar] [CrossRef]
- Salmeia, K.A.; Gaan, S. An overview of some recent advances in DOPO-derivatives: Chemistry and flame retardant applications. Polym. Degrad. Stab. 2015, 113, 119–134. [Google Scholar] [CrossRef]
- Vasiljević, J.; Čolović, M.; Čelan Korošin, N.; Šobak, M.; Štirn, Ž.; Jerman, I. Effect of Different Flame-Retardant Bridged DOPO Derivatives on Properties of in Situ Produced Fiber-Forming Polyamide 6. Polymers 2020, 12, 657. [Google Scholar] [CrossRef]
- White, K.M.; Angell, Y.L.; Angell, S.E.; Mack, A.G. Dopo-Derived Flame Retardant and Epoxy Resin Composition. WO2010135393A1, 25 November 2010. [Google Scholar]
- Shree Meenakshi, K.; Pradeep Jaya Sudhan, E.; Ananda Kumar, S.; Umapathy, M.J. Development and characterization of novel DOPO based phosphorus tetraglycidyl epoxy nanocomposites for aerospace applications. Prog. Org. Coat. 2011, 72, 402–409. [Google Scholar] [CrossRef]
- Lin, C.H.; Huang, C.M.; Wang, M.W.; Dai, S.A.; Chang, H.C.; Juang, T.Y. Synthesis of a Phosphinated Acetoxybenzoic Acid and Its Application in Enhancing Tg and Flame Retardancy of Poly(ethylene terephthalate). J. Polym. Sci. Pol. Chem. 2014, 52, 424–434. [Google Scholar] [CrossRef]
- Lin, Y.; Jiang, S.; Gui, Z.; Li, G.; Shi, X.; Chen, G.; Peng, X. Synthesis of a novel highly effective flame retardant containing multivalent phosphorus and its application in unsaturated polyester resins. RSC Adv. 2016, 6, 86632–86639. [Google Scholar] [CrossRef]
- Wang, H.; Wang, S.; Du, X.; Wang, H.; Cheng, X.; Du, Z. Synthesis of a novel flame retardant based on DOPO derivatives and its application in waterborne polyurethane. RSC Adv. 2019, 9, 7411–7419. [Google Scholar] [CrossRef]
- Chen, Y.K.; Lu, Q.X.; Zhong, G.; Zhang, H.G.; Chen, M.F.; Liu, C.P. DOPO-based curing flame retardant of epoxy composite material for char formation and intumescent flame retardance. J. Appl. Polym. Sci. 2021, 138, 49918. [Google Scholar] [CrossRef]
- Wang, C.-S.; Shieh, J.-Y. Synthesis and properties of epoxy resins containing 2-(6-oxid-6H-dibenz<c,e><1,2>oxaphosphorin-6-yl)1,4-benzenediol. Polymer 1998, 39, 5819–5826. [Google Scholar] [CrossRef]
- Bai, Z.; Song, L.; Hu, Y.; Yuen, R.K.K. Preparation, flame retardancy, and thermal degradation of unsaturated polyester resin modified with a novel phosphorus containing acrylate. Ind. Eng. Chem. Res. 2013, 52, 12855–12864. [Google Scholar] [CrossRef]
- Kishimoto, D.; Umeki, Y. High Melting Point Flame Retardant Crystal and Method for Manufacturing the Same, Epoxy Resin Composition Containing the Flame Retardant, and Prepreg and Flame Retardant Laminate Using the Composition. US20130053473A1, 28 February 2013. [Google Scholar]
- Liu, P.; Liu, M.; Gao, C.; Wang, F.; Ding, Y.; Wen, B.; Zhang, S.; Yang, M. Preparation, characterization and properties of a halogen-free phosphorous flame-retarded poly(butylene terephthalate) composite based on a DOPO derivative. J. Appl. Polym. Sci. 2013, 130, 1301–1307. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, S.M.; Zhao, J.Q.; Huang, J.Y. Synthesis and properties of a modified unsaturated polyester resin with phosphorus-containing pendant groups. Polym. Bull. 2013, 70, 1097–1111. [Google Scholar] [CrossRef]
- Dittrich, U.; Just, B.; Döring, M.; Ciesielski, M. Process for the Preparation of 9,10-dihydro-9-oxa-10-organylphosphaphenanthrene-10-oxide and Derivatives of the Same Substituted on the Phenyl Groups. US20050038279A1, 17 February 2005. [Google Scholar]
- Artner, J.; Ciesielski, M.; Ahlmann, M.; Walter, O.; Döring, M.; Perez, R.M.; Altstädt, V.; Sandler, J.K.W.; Schartel, B. A Novel and Effective Synthetic Approach to 9,10-Dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) Derivatives. Phosphorus Sulfur 2007, 182, 2131–2148. [Google Scholar] [CrossRef]
- Koenig, A.; Kroke, E. Flame retardancy working mechanism of methyl-DOPO and MPPP in flexible polyurethane foam. Fire Mater. 2012, 36, 1–15. [Google Scholar] [CrossRef]
- Wagner, S.; Rakotomalala, M.; Bykov, Y.; Walter, O.; Döring, M. Synthesis of new organophosphorus compounds using the atherton–todd reaction as a versatile tool. Heteroatom. Chem. 2012, 23, 216–222. [Google Scholar] [CrossRef]
- Buczko, A.; Stelzig, T.; Bommer, L.; Rentsch, D.; Heneczkowski, M.; Gaan, S. Bridged DOPO derivatives as flame retardants for PA6. Polym. Degrad. Stabil. 2014, 107, 158–165. [Google Scholar] [CrossRef]
- Le Corre, S.S.; Berchel, M.; Couthon-Gourvès, H.; Haelters, J.P.; Jaffrès, P.-A. Atherton–Todd reaction: Mechanism, scope and applications. Beilstein J. Org. Chem. 2014, 10, 1166–1196. [Google Scholar] [CrossRef] [PubMed]
- Jian, R.; Wang, P.; Duan, W.; Wang, J.; Zheng, X.; Weng, J. Synthesis of a Novel P/N/S-Containing Flame Retardant and Its Application in Epoxy Resin: Thermal Property, Flame Retardance, and Pyrolysis Behavior. Ind. Eng. Chem. Res. 2016, 55, 11520–11527. [Google Scholar] [CrossRef]
- Stelzig, T.; Bommer, L.; Gaan, S.; Buczko, A. DOPO-Based Hybrid Flame Retardants. US20170081590A1, 23 March 2017. [Google Scholar]
- Zhang, Y.; Yu, B.; Wang, B.; Meow Liew, K.; Song, L.; Wang, C.; Hu, Y. Highly Effective P–P Synergy of a Novel DOPO-Based Flame Retardant for Epoxy Resin. Ind. Eng. Chem. Res. 2017, 56, 1245–1255. [Google Scholar] [CrossRef]
- Gaan, S.; Neisius, M.; Mercoli, P.; Liang, S.; Mispreuve, H.; Näscher, R. Novel Phosphonamidates-Synthesis and Flame Retardant Application. WO2013020696A2, 14 February 2013. [Google Scholar]
- Neisius, N.M.; Lutz, M.; Rentsch, D.; Hemberger, P.; Gaan, S. Synthesis of DOPO-Based Phosphonamidates and their Thermal Properties. Ind. Eng. Chem. Res. 2014, 53, 2889–2896. [Google Scholar] [CrossRef]
- Salmeia, K.A.; Baumgartner, G.; Jovic, M.; Gössi, A.; Riedl, W.; Zich, T.; Gaan, S. Industrial Upscaling of DOPO-Based Phosphonamidates and Phosphonates Derivatives Using Cl2 Gas as a Chlorinating Agent. Org. Process Res. Dev. 2018, 22, 1570–1577. [Google Scholar] [CrossRef]
- Salmeia, K.A.; Flaig, F.; Rentsch, D.; Gaan, S. One-Pot Synthesis of P(O)-N Containing Compounds Using N-Chlorosuccinimide and Their Influence in Thermal Decomposition of PU Foams. Polymers 2018, 10, 740. [Google Scholar] [CrossRef]
- Salmeia, K.A.; Gooneie, A.; Simonetti, P.; Nazir, R.; Kaiser, J.-P.; Rippl, A.; Hirsch, C.; Lehner, S.; Rupper, P.; Hufenus, R.; et al. Comprehensive study on flame retardant polyesters from phosphorus additives. Polym. Degrad. Stabil. 2018, 155, 22–34. [Google Scholar] [CrossRef]
- Natchev, I.A. Three-component condensation of ω-hydroxy-L-α-aminocarboxylic acids, water and phosphorus trichloride or methyldichlorophosphine. Phosphorus Sulfur 1988, 37, 149–157. [Google Scholar] [CrossRef]
- Enders, D.; Saint-Dizier, A.; Lannou, M.I.; Lenzen, A. The Phospha-Michael Addition in Organic Synthesis. Eur. J. Org. Chem. 2006, 29–49. [Google Scholar] [CrossRef]
- Lenz, J.; Pospiech, D.; Komber, H.; Paven, M.; Albach, R.; Mentizi, S.; Langstein, G.; Voit, B. Synthesis of the H-phosphonate dibenzo[d,f][1,3,2]dioxaphosphepine 6-oxide and the phospha-Michael addition to unsaturated compounds. Tetrahedron 2019, 75, 1306–1310. [Google Scholar] [CrossRef]
- Leu, T.-S.; Wang, C.-S. Synergistic Effect of a Phosphorus–Nitrogen Flame Retardant on Engineering Plastics. J. Appl. Polym. Sci. 2004, 92, 410–417. [Google Scholar] [CrossRef]
- Li, Q.; Jiang, P.; Su, Z.; Wei, P.; Wang, G.; Tang, X. Synergistic effect of phosphorus, nitrogen, and silicon on flame-retardant properties and char yield in polypropylene. J. Appl. Polym. Sci. 2005, 96, 854–860. [Google Scholar] [CrossRef]
- Gaan, S.; Sun, G.; Hutches, K.; Engelhard, M.H. Effect of nitrogen additives on flame retardant action of tributyl phosphate: Phosphorus–nitrogen synergism. Polym. Degrad. Stab. 2008, 93, 99–108. [Google Scholar] [CrossRef]
- Nguyen, C.; Kim, J. Synthesis of a novel nitrogen-phosphorus flame retardant based on phosphoramidate and its application to PC, PBT, EVA, and ABS. Macromol. Res. 2008, 16, 620–625. [Google Scholar] [CrossRef]
- Bauer, K.N.; Tee, H.T.; Velencoso, M.M.; Wurm, F.R. Main-chain poly(phosphoester)s: History, syntheses, degradation, bio-and flame-retardant applications. Prog. Polym. Sci. 2017, 73, 61–122. [Google Scholar] [CrossRef]
- Sykam, K.; Kumar Reddy Meka, K.; Donempudi, S. Intumescent Phosphorus and Triazole-Based Flame-Retardant Polyurethane Foams from Castor Oil. ACS Omega 2019, 4, 1086–1094. [Google Scholar] [CrossRef]
- Bortoluzzi, M.; Gobbo, A. 1,3-Dimethyl-2-phenyl-1,3-diazaphospholidine-2-oxide as ligand for the preparation of luminescent lanthanide complexes. J. Coord. Chem. 2019, 72, 1524–1536. [Google Scholar] [CrossRef]
- Bortoluzzi, M.; Castro, J.; Gobbo, A.; Ferraro, V.; Pietrobon, L.; Antoniutti, S. Tetrahedral photoluminescent manganese(II) halide complexes with 1,3-dimethyl-2-phenyl-1,3-diazaphospholidine-2-oxide as a ligand. New J. Chem. 2020, 44, 571–579. [Google Scholar] [CrossRef]
- Bortoluzzi, M.; Di Vera, A.; Pietrobon, L.; Castro, J. Seven- and eight-coordinate lanthanide(III) amidophosphate complexes: Synthesis, characterization and photoluminescence. J. Coord. Chem. 2021, 74, 1466–1481. [Google Scholar] [CrossRef]
- Bortoluzzi, M.; Castro, J.; Di Vera, A.; Palù, A.; Ferraro, V. Manganese(II) bromo- and iodo-complexes with phosphoramidate and phosphonate ligands: Synthesis, characterization and photoluminescence. New J. Chem. 2021, 45, 12871–12878. [Google Scholar] [CrossRef]
- Ferraro, V.; Castro, J.; Agostinis, L.; Bortoluzzi, M. Dual-emitting Mn(II) and Zn(II) halide complexes with 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide as ligand. Inorg. Chim. Acta 2023, 545, 121285. [Google Scholar] [CrossRef]
- Agostinis, L.; Ghincolov, S.; Bortoluzzi, M. Preparation Process of P(=O)-Heteroatom Derivatives of Dibenzooxaphosphacycles. WO2023094526A1, 1 June 2023. [Google Scholar]
- Panmand, D.S.; Tiwari, A.D.; Panda, S.S.; Monbaliu, J.-C.M.; Beagle, L.K.; Asiri, A.M.; Stevens, C.V.; Steel, P.J.; Hall, C.D.; Katritzky, A.R. New benzotriazole-based reagents for the phosphonylation of various N-, O-, and S-nucleophiles. Tetrahedron Lett. 2014, 55, 5898–5901. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).