Abstract
Phthalate esters are used in colognes and other cosmetics and related products to maintain fragrance. Additionally, phthalates can dissolve and stabilize certain aroma ingredients and essential oils. The results of a five-year study (2016–2020) conducted in a quality control laboratory on the presence of phthalate esters in various cosmetic products are presented. A total of 1147 samples from four cologne categories: eau de toilette, eau de cologne, fragrance, and perfume were analyzed using gas chromatography–mass spectrometry (GC-MS/MS) for the quantification of the nine different phthalate esters (BBP, DEHP, DNOP, DNPP, DBP, DIPP, DMEP, DMP, and PIPP) in each category. The results revealed the absence of phthalates at concentrations above the threshold limit (1 µg mL−1) in 95% of the samples analyzed. However, different levels of phthalates were detected in 57 samples, mainly DEHP and DBP. Our findings also demonstrate distinct phthalate profiles according to cologne type, with relevant variations among the four cologne categories analyzed.
1. Introduction
Cosmetic products consist of substances or combinations of substances designed for contact with the outer layers of the human body (such as the epidermis, hair, nails, etc.) as well as teeth and oral mucous membranes. Cosmetics are used for the primary purposes of cleansing, perfuming, altering appearance, protecting, maintaining or correcting body odors. Due to their usage, products like colognes, perfumes, or fragrances expose humans to their components, potentially causing skin reactions, allergies, or other adverse effects. Some research has suggested that specific phthalates might lead to negative health impacts, such as endocrine disruption and reproductive toxicity [1,2]. Phthalates belong to a category of chemical compounds employed in the production of scented items like colognes and perfumes for various reasons [3,4]. Phthalates aid in maintaining and stabilizing fragrances within products, ensuring that the scent remains consistent and does not dissipate rapidly after application. The use of phthalates in the formulation can slow down the evaporation of volatile fragrance components, contributing to a longer-lasting scent on the skin. Moreover, these compounds facilitate the blending of essential oils and other fragrance ingredients, ensuring even distribution in the cologne solution.
Governments and regulatory bodies must ensure and guarantee the safety of cosmetic products, including colognes, fragrances, and perfumes. Therefore, regulatory agencies around the world place restrictions on the use of phthalates in cosmetic and personal care products. These regulations vary by country but generally include limitations on phthalate concentrations and labeling requirements. In the case of the European Union, EC regulation 1223/2009 details the prohibited or restricted substances in cosmetics, in addition to indicating the labeling requirements for these products [5]. Among the different categories of chemical products used as additives in perfumes or colognes, European regulations subject phthalates to especially strict monitoring. Six phthalates (dibutyl phthalate (DBP), bis(2-methoxyethyl) phthalate (DMEP), diisopentyl phthalate (DIPP), di-n-pentyl phthalate (DNPP), benzyl butyl phthalate (BBP), and bis(2-ethylhexyl) phthalate (DEHP), have been banned as ingredients in cosmetics and personal care products due to their possible carcinogenic and mutagenic effects on human health.
In this paper, we present the preliminary results of a five-year quality control screening (2016–2020) for phthalate content using GC-MS/MS in a large database of colognes, which includes 1147 samples, encompassing eau de cologne, eau de toilette, fragrances, and perfumes.
2. Material and Methods
2.1. Samples Collection and Sample Preparation
In this study, a total of 1147 cosmetic samples were analyzed in a quality control laboratory specialized in textiles, cosmetics, and related materials during the period of 2016–2020. The samples belong to four different categories: eau de cologne (n = 41), eau de toilette (n = 789), fragrance (n = 87), and perfume (n = 230). In all cases, samples were sourced directly from the manufacturers or suppliers in their original and properly sealed containers. They were stored in a dark and temperature-controlled environment until analysis. Most of the samples are of Spanish origin, but the sample set also included a significant number from France and Sweden.
2.2. Reagents and Apparatus
All solvents used were of chromatography grade and were supplied by Merck (Darmstadt, Germany). Ultra-pure water was produced using a Milli-Q system (Millipore, Bedford, MA, USA). Standards for the different phthalate esters analyzed were obtained from LGC Group (Middlesex, UK), Sigma-Aldrich (Madrid, Spain), and Scharlab (Barcelona, Spain). Deuterated di-n-octyl phthalate (DNOP-d4) and deuterated bis-(2-ethylhexyl) phthalate (DEHP-d4), both used as internal standards, were provided by Laboratorios CIFGA S.A. (Lugo, Spain). Anthracene-d10, also used as an internal standard, was purchased from Sigma-Aldrich (Vallensbaek Strand, Denmark). Phthalate determinations were carried out using an Agilent 7890B gas chromatograph coupled to a 7000 Series Triple Quad Mass Spectrometer detector (GC-MS/MS) from Agilent Technologies (Agilent, Santa Clara, CA, USA).
2.3. Analytical Procedure
Phthalates in cosmetics, specifically in perfume, were determined following the British Standard EN 16521:2014 [6]. Samples were prepared for analysis using the following procedure: 1.0 mL of the liquid sample was diluted with ethyl acetate to a total volume of 10 mL in a volumetric flask. The resulting solution was then filtered through a 0.45 μm syringe filter. Subsequently, 980 µL of this sample solution was mixed with 20 µL of a 50 mg L−1 internal standard working solution and injected into the GC-MS/MS system. The analytes present in the sample were evaporated in the inlet of the gas chromatograph at high temperatures and introduced into the chromatographic column. Separation of the analytes occurred on a fused silica capillary column HP-5MS (30 m × 0.25 mm ID, 0.25 μm) using a temperature gradient program. The analytes were then separated and introduced into the electron impact ion source of the mass spectrometry. The ions generated were introduced into the mass spectrometer analyzer, where they were selected based on the m/z ratio and subsequently quantified using a detector. Signal acquisition by the GC-MS/MS equipment was carried out in MRM mode. The working conditions used in the analysis are detailed in Table 1.

Table 1.
GC-MS/MS experimental conditions.
The analytes were quantified by comparing them to an external calibration curve while correcting for the signal of the internal standard. The identification of the analytes was carried out by comparing the samples against the external calibration standards based on the relative signal obtained for the selected characteristic ions using the MRM detection mode. Following this procedure, nine phthalate esters were determined: benzyl butyl phthalate (BBP), bis(2-ethylhexyl) phthalate (DEHP), di(n-octyl) phthalate (DNOP), di-n-pentyl phthalate (DNPP), dibutyl phthalate (DBP), diisopentyl phthalate (DIPP), bis(2-methoxyethyl) phthalate (DMEP), dimethyl phthalate (DMP), and isopentyl pentyl phthalate (PIPP).
The analytical measurements underwent a stringent quality control process throughout the measurement period to ensure the reliability of the obtained results.
3. Results and Discussion
The samples from the four categories (eau de cologne, eau de toilette, fragrance, and perfume) were analyzed for the content of the nine phthalates, as outlined in Section 2.3. For the 1147 samples measured, phthalate esters were only detected in concentrations higher than the LOD (1 µg mL−1) in 57 samples, which represents 4.97% of the total. In 39 of these samples, only one phthalate was detected, while in the remaining 18, the simultaneous presence of two different phthalates was observed. None of the samples showed the presence of more than two phthalates. A summary of the positive analyzed samples (with phthalate content exceeding 1 µg mL−1) can be found in Table 2. Depending on the type of samples analyzed, the number of positive results in each category was as follows: no sample showed any phthalate content in eau de cologne, 22 samples indicated the presence of phthalates in eau de toilette, nine samples in fragrance, and 26 in perfume, with the latter equally likely to have one or two phthalates detected. The percentage of positive samples in each category varies, increasing from the eau de cologne category, where no positive samples were detected, to the perfume category, where 11.30% of the samples exhibited some PAE.

Table 2.
Summary of the analyzed samples.
The frequency of the occurrence of different PAEs is summarized in Table 3. DEHP is the PAE that appears in the highest number of positive samples, with a total of 43 appearances (25 of them in samples containing a single PAE and 18 in samples containing two PAEs). DBP is the second most frequently detected phthalate, present in 22 out of the 57 positive samples (in 5 samples as the sole PAE and in 17 other samples containing two PAEs).

Table 3.
Distribution of the number and type of PAEs in the positive samples.
DMP and DMEP are the other two PAEs detected in the analyzed samples, although their occurrence is much lower compared to DEHP and DBP. Specifically, DMP was detected in six positive samples, while DMEP was only found in four samples. It is noteworthy that in the 18 positive samples in which two different phthalates were detected, the combination in all cases was DEHP-DBP, except for one instance of DEHP-DMEP. The remaining PAEs considered (BBP, DNOP, DNPP, DIPP, and PIPP) were not detected in any of the samples analyzed.
Furthermore, when considering the percentage of positive samples within each category, a direct correlation is observed with the concentration of aromatic essence in each product. The percentage of positive samples relative to the total number of samples analyzed in each category is as follows: 0% for the eau de cologne category, 2.79% for the eau de toilette category, 10.34% for the fragrance category, and 11.30% in the case of the perfume category. This phenomenon can be explained by considering that the addition of PAEs allows for the fixation of aromas produced by the essences contained in the product. The essence content increases from eau de cologne (2–5%), eau de toilette (5–15%), and fragrance (15–20%), up to the highest value in perfume (20–30%). Additionally, this justifies the observation that the majority of samples containing two PAEs are fragrances (33.33%), especially perfumes (61.11%).
The concentration levels for each PAE in the 57 positive samples from the four categories are summarized in Table 4. As shown, DEHP is not only the PAE with the highest frequency of appearance but also the one found in the highest concentration, an order of magnitude higher than any other detected PAE. When considering different types of products, it is also observed (see Figure 1) that DEHP concentrations are higher in categories with a higher percentage of aromatic essence. Consequently, higher concentrations were measured in perfumes, followed by fragrances and eau de cologne. A similar concentration pattern was observed for DBP. The other two detected phthalates (DMEP and DMP) were not commented upon due to the small number of samples in which these PAEs appeared. The results obtained presented comparable levels to those reported by Koniecki et al. [7] in fragrance samples and by Al-Saleh and Elkhatib [8] in branded perfumes. However, in the latter case, the frequency of appearance was reported as BBP > DMP > DEP > DEHP > DBP (using a different threshold limit of 0.1 µg mL−1 for BBP, DMP, and DBP).

Table 4.
Distribution of the number and type of PAEs in the positive samples.
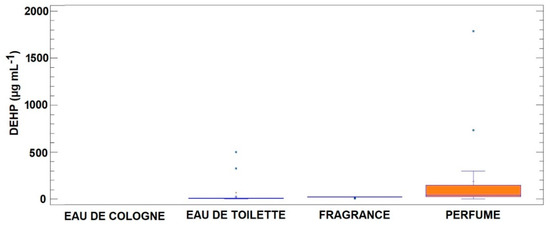
Figure 1.
Box and whisker plot for the concentrations of DEHP according to the different categories considered in this study. All concentrations are in µg L−1.
In addition, ANOVA studies were conducted to assess the potential impact of the sample’s origin and the year of analysis on phthalate content. The results indicated that there are no significant differences (at a 95% confidence level) in the concentration of phthalates with respect to the country of origin and the year of analysis.
4. Conclusions
The results of a quality control system applied for five years to verify the phthalate content of a large set of samples from different types of colonies yielded the following conclusions. GC-MS/MS has been demonstrated as an appropriate analytical technique for the measurement of phthalates in cosmetic samples, particularly in different types of colonies. Moreover, the percentage of non-compliant samples with a total phthalate content greater than 1 µg mL−1 was 4.97% of the total samples analyzed. These batches must be reprocessed before being marketed, with the consequent economic cost. In most of the samples, a single phthalate was detected, which was DEHP in most cases. The samples in which two phthalates appear correspond to the categories with the highest concentration of aromatic essence: fragrance and perfumes, which, in turn, also present the highest concentrations.
Finally, it is clear that the control of phthalates in cosmetic products is relevant to guaranteeing consumer safety, ensuring compliance with legal regulations, ensuring product quality and contributing to increasing transparency and informed use of cosmetics.
Author Contributions
Conceptualization, N.A.-L. and C.H.-L.; methodology, N.A.-L.; validation, N.A.-L. and C.H.-L.; formal analysis, N.A.-L., M.L.-A. and C.H.-L.; investigation, N.A.-L.; resources, M.L.-A. and C.H.-L.; data curation, C.H.-L.; writing—original draft preparation, N.A.-L. and C.H.-L.; writing—review and editing, N.A.-L. and C.H.-L.; supervision, M.L.-A. and C.H.-L.; project administration, C.H.-L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kazemi, Z.; Aboutaleb, E.; Shahsavani, A.; Kermani, M.; Kazemi, Z. Evaluation of pollutants in perfumes, colognes and health effects on the consumer: A systematic review. J. Environ. Health. Sci. Eng. 2022, 20, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Chisvert, A.; López-Nogueroles, M.; Miralles, P.; Salvador, A. Perfumes in cosmetics: Regulatory aspects and analytical methods (Chapter 10). In Analysis of Cosmetic Products, 2nd ed.; Salvador, A., Chisvert, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 225–248. [Google Scholar] [CrossRef]
- Sanchez-Prado, L.; Llompart, M.; Lamas, J.P.; García-Jares, C.; Lores, M. Multicomponent analytical methodology to control phthalates, synthetic musks, fragrance allergens and preservatives in perfumes. Talanta 2011, 85, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Celeiro, M.; Lamas, J.P.; Llompart, M.; García-Jares, C. In-vial micro-matrix solid phase dispersion for the analysis of fragrance allergens, preservatives, plasticizers and musks in cosmetics. Cosmetics 2014, 1, 171–201. [Google Scholar] [CrossRef]
- European Union. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products (recast). Off. J. Eur. Union 2009, L342, 59–209. Available online: https://health.ec.europa.eu/system/files/2016-11/cosmetic_1223_2009_regulation_en_0.pdf (accessed on 1 July 2023).
- EN 16521:2014; Cosmetics, Analytical Methods. GC/MS Method for the Identification and Assay of 12 Phthalates in Cosmetic Samples Ready for Analytical Injection. European Committee for Standardization: Brussels, Belgium, 2014.
- Koniecki, D.; Wang, R.; Moody, R.P.; Zhu, J. Phthalates in cosmetic and personal care products: Concentrations and possible dermal exposure. Environ. Res. 2011, 111, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Al-Saleh, I.; Elkhatib, R. Screening of phthalate esters in 47 branded perfumes. Environ. Sci. Pollut. Res. 2016, 23, 455–468. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).