Organocatalytic Properties of 3,4-Dihydroxyprolines †
Abstract
:1. Introduction
2. Result and Discussion
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eder, U.; Sauer, G.; Wiechert, R. New type of asymmetric cyclization to optically active steroid CD partial structures. Angew. Chem. Int. Ed. 1971, 10, 496–497. [Google Scholar] [CrossRef]
- Hajos, Z.G.; Parrish, D.R. Asymmetric synthesis of bicyclic intermediates of natural product chemistry. J. Org. Chem. 1974, 39, 1615–1621. [Google Scholar] [CrossRef]
- List, B.; Lerner, R.A.; Barbas, C.F., III. Proline-catalyzed direct asymmetric aldol reactions. J. Am. Chem. Soc. 2000, 122, 2395–2396. [Google Scholar] [CrossRef]
- Ahrendt, K.A.; Borths, C.J.; MacMillan, D.W.C. New strategies for organic catalysis: The first highly enantioselective organocatalytic Diels−Alder reaction. J. Am. Chem. Soc. 2000, 122, 4243–4244. [Google Scholar] [CrossRef]
- Trost, B.M.; Brindle, C.S. The direct catalytic asymmetric aldol reaction. Chem. Soc. Rev. 2010, 39, 1600–1632. [Google Scholar] [CrossRef] [PubMed]
- Vishumaya, M.R.; Singh, V.K. Highly efficient small organic molecules for enantioselective direct aldol reaction in organic and aqueous media. J. Org. Chem. 2009, 74, 4289–4297. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Sumiya, T.; Takahashi, J.; Gotoh, H.; Urushima, T.; Shoji, M. Highly diastereo-and enantioselective direct aldol reactions in water. Angew. Chem. Int. Ed. 2006, 45, 958–961. [Google Scholar] [CrossRef] [PubMed]
- Aratake, S.; Itoh, T.; Okano, T.; Nagae, N.; Sumiya, T.; Shoji, M.; Hayashi, Y. Highly Diastereo- and Enantioselective Direct Aldol Reactions of Aldehydes and Ketones Catalyzed by Siloxyproline in the Presence of Water. Chemistry 2007, 13, 10246–10256. [Google Scholar] [CrossRef] [PubMed]
- Córdova, A.; Zou, W.; Dziedzic, P.; Ibrahem, I.; Reyes, E.; Xu, Y. Direct asymmetric intermolecular aldol reactions catalyzed by amino acids and small peptides. Chem. Eur. J. 2006, 12, 5383–5397. [Google Scholar] [CrossRef] [PubMed]
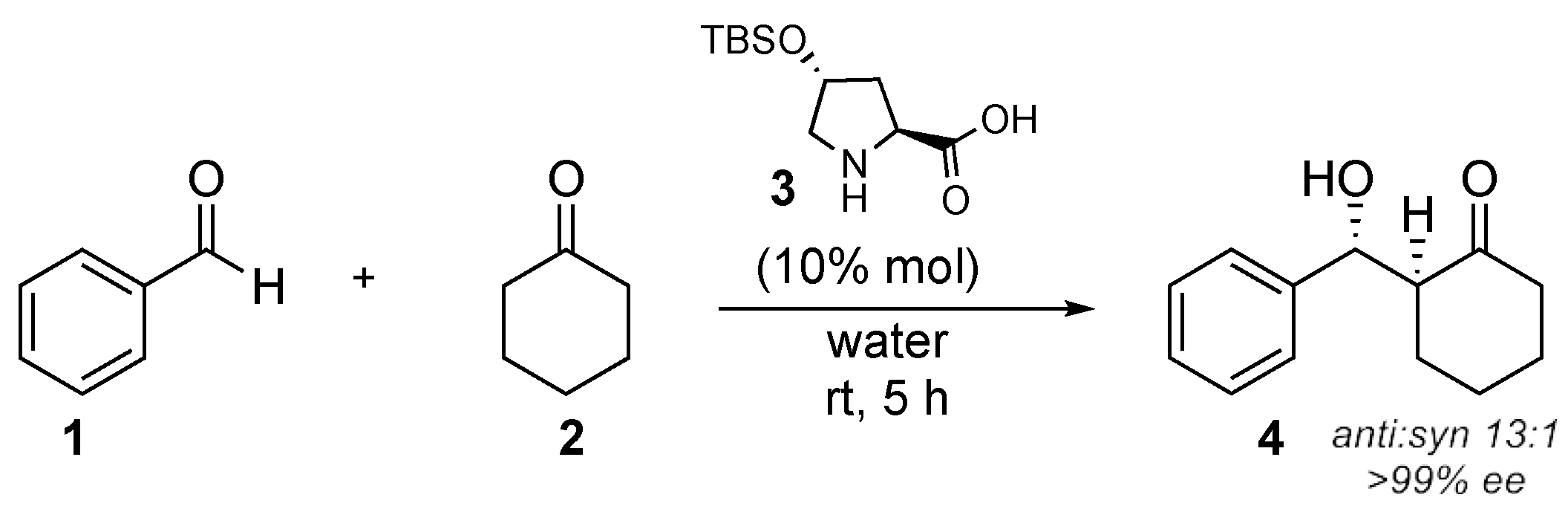
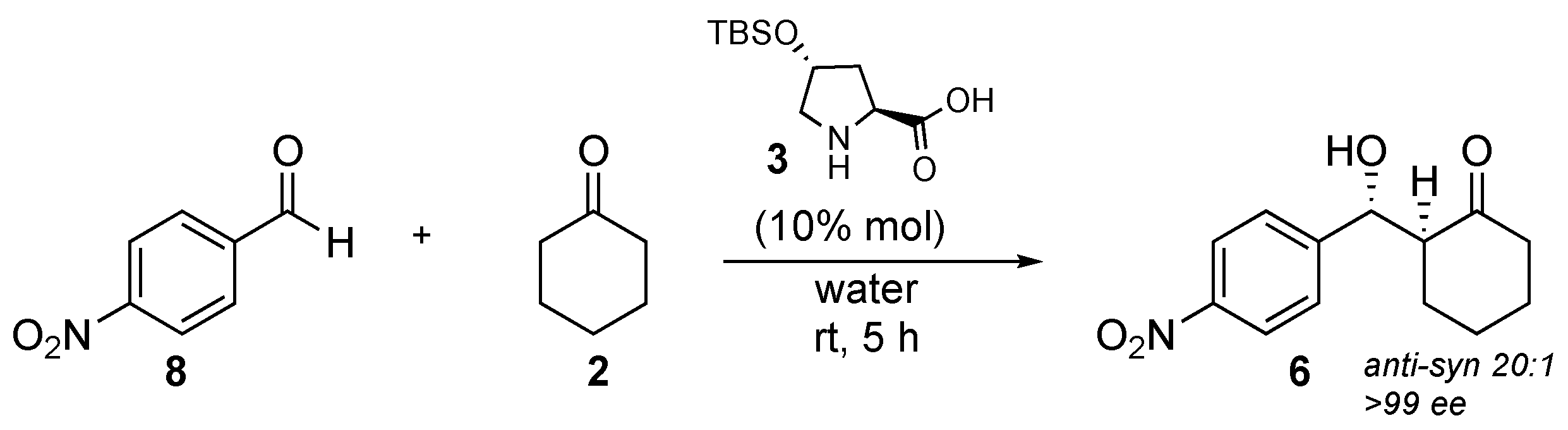
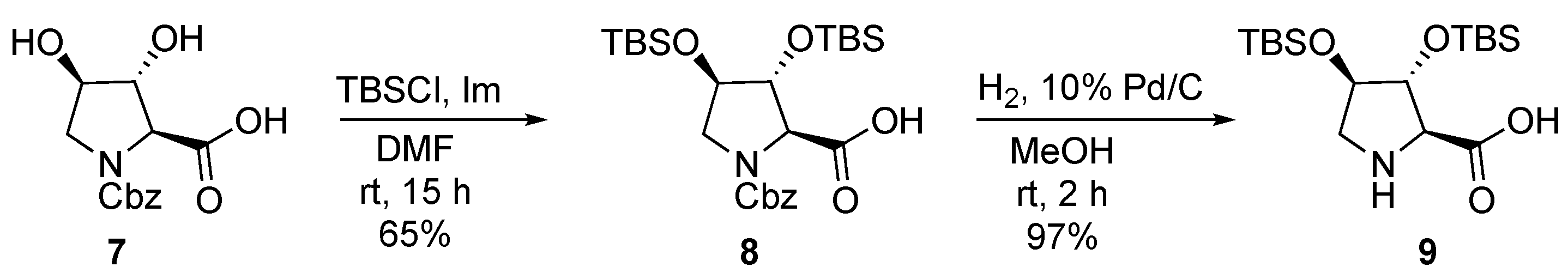
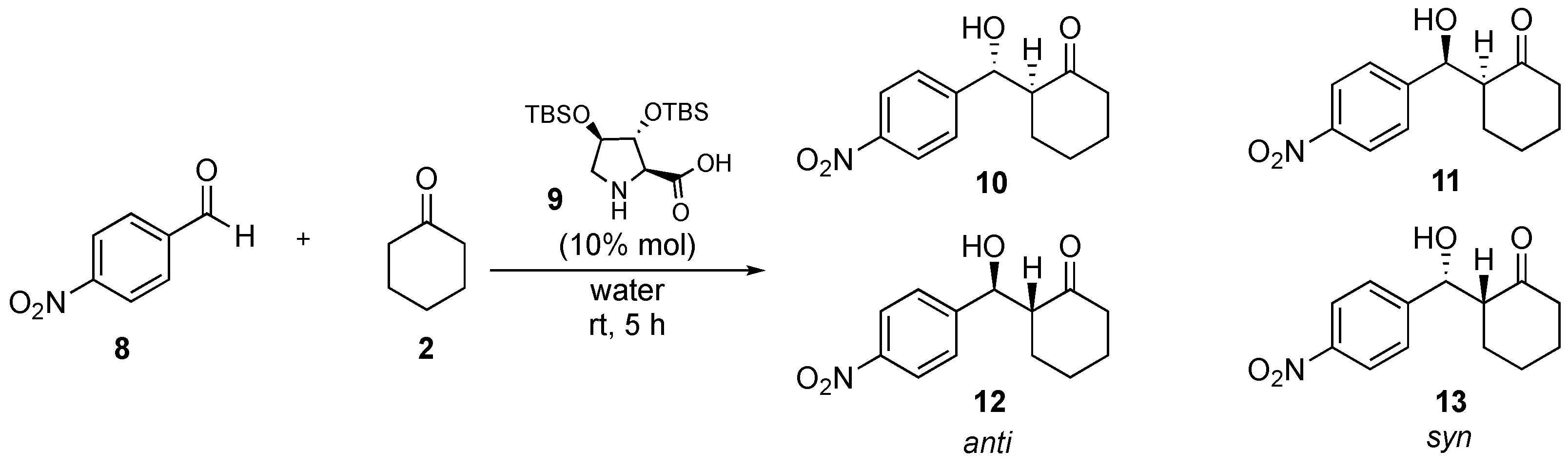
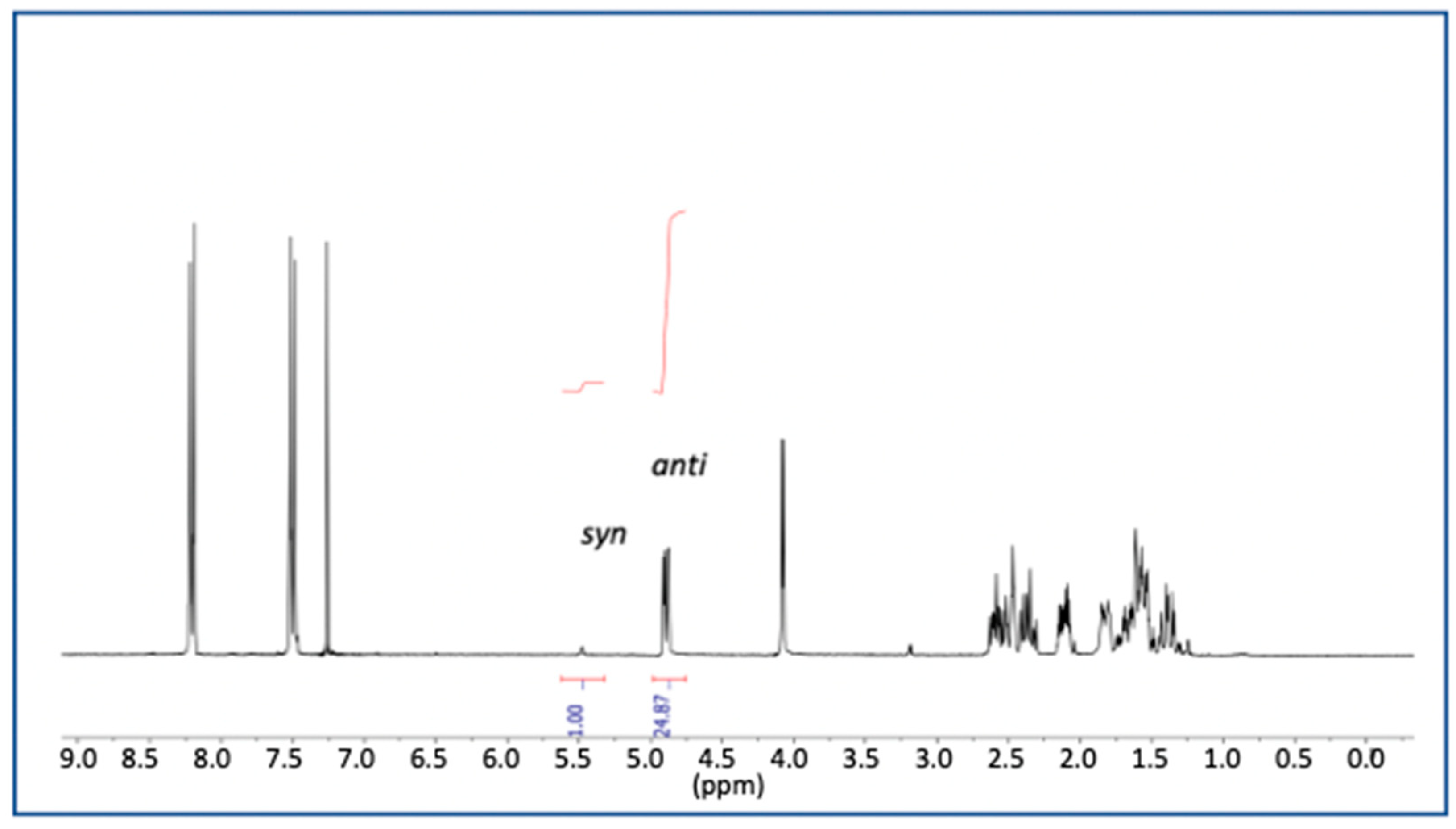
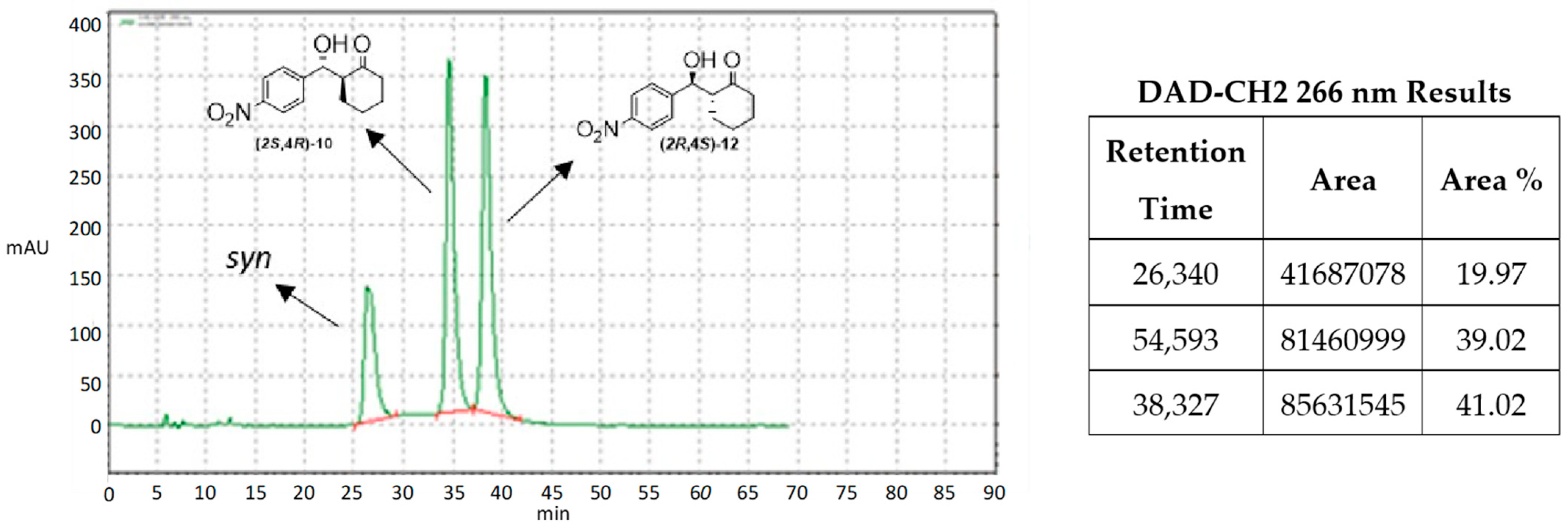
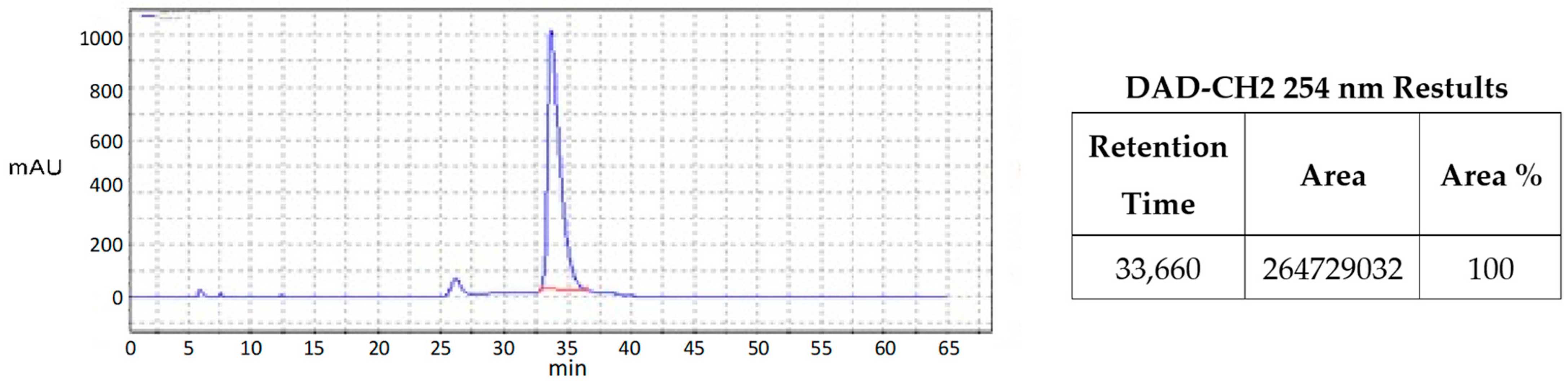
| Catalyst | Overall Yield | e.e. | Anti/syn Ratio | Major Reaction Product |
|---|---|---|---|---|
| 3 | 86% | >99% | 20:1 | 10 |
| 9 | 86% | >99% | 25:1 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estévez, R.J.; Balo, R.; Fernández, A.; Estévez, J.C. Organocatalytic Properties of 3,4-Dihydroxyprolines. Chem. Proc. 2023, 14, 107. https://doi.org/10.3390/ecsoc-27-16117
Estévez RJ, Balo R, Fernández A, Estévez JC. Organocatalytic Properties of 3,4-Dihydroxyprolines. Chemistry Proceedings. 2023; 14(1):107. https://doi.org/10.3390/ecsoc-27-16117
Chicago/Turabian StyleEstévez, Ramón J., Rosalino Balo, Andrés Fernández, and Juan C. Estévez. 2023. "Organocatalytic Properties of 3,4-Dihydroxyprolines" Chemistry Proceedings 14, no. 1: 107. https://doi.org/10.3390/ecsoc-27-16117
APA StyleEstévez, R. J., Balo, R., Fernández, A., & Estévez, J. C. (2023). Organocatalytic Properties of 3,4-Dihydroxyprolines. Chemistry Proceedings, 14(1), 107. https://doi.org/10.3390/ecsoc-27-16117







