One-Pot Synthesis of Tetrazole–Triazole Bis-Heterocycles via Ugi–Azide Reaction †
Abstract
:1. Introduction
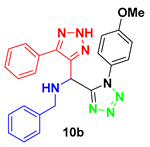
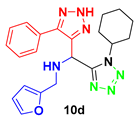
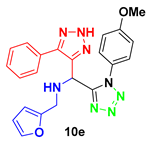
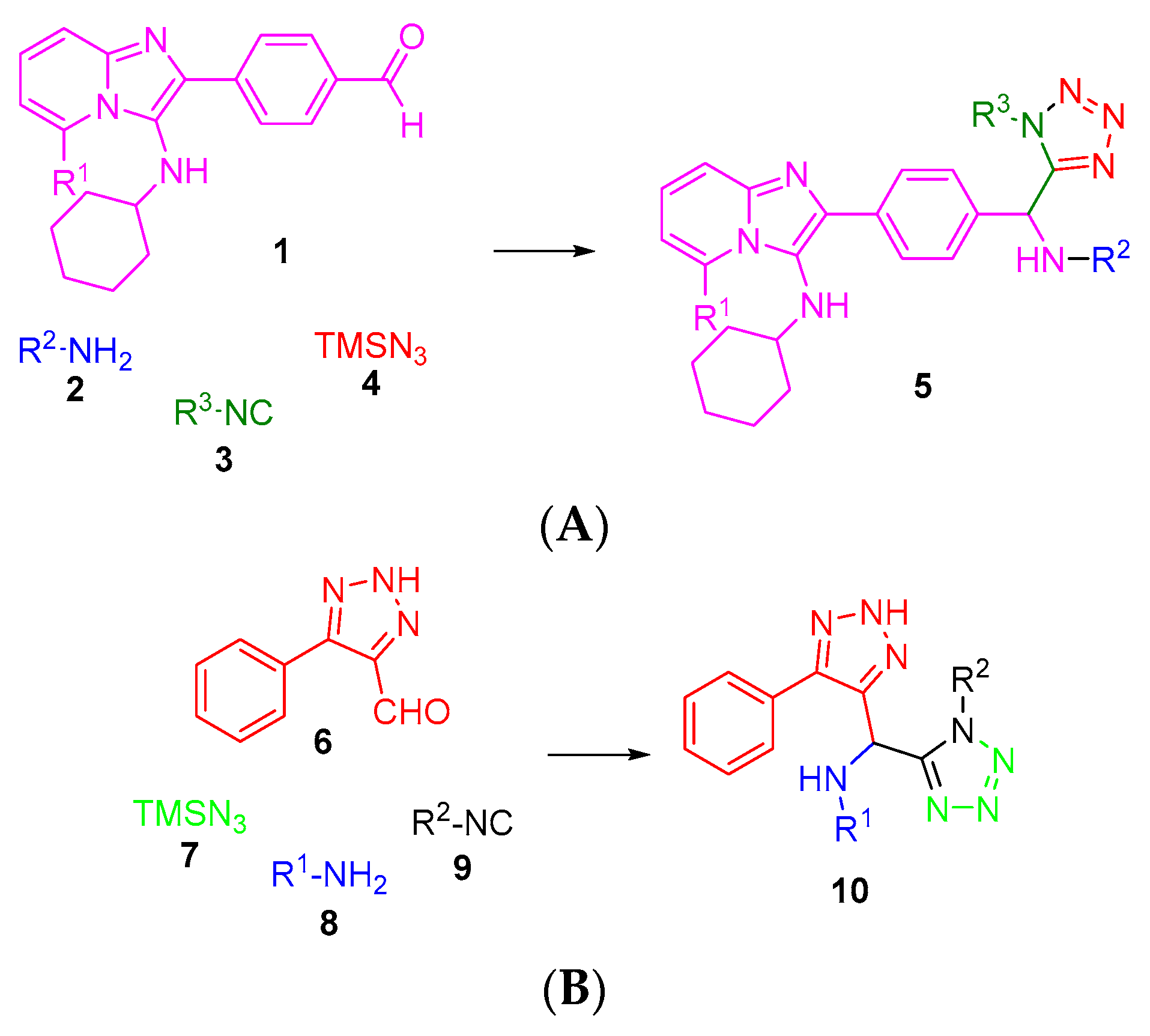
2. Results and Discussion
3. Conclusions
4. Experimental Section
4.1. General information, Chemicals, and Instrumentation
4.2. General Procedure
4.3. Spectral Data


Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ruijter, E.; Orru, R.V.A. Discovery of MCRs. In Multicomponent Reactions in Organic Synthesis, 1st ed.; Zhu, J., Wang, Q., Wang, M., Eds.; Wiley-VCH: Weinheim, Germany, 2015; pp. 13–15. [Google Scholar]
- Tietze, L.F.; Brasche, G.; Gericke, K.M. Domino Reactions in Organic Synthesis, 1st ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006; p. 542. [Google Scholar]
- Ugi, I.; Dömling, A.; Ebert, B. Combinatorial Chemistry of Multicomponent Reactions. In Combinatorial Chemistry: Synthesis, Analysis, Screening, 1st ed.; Günther, J., Ed.; WYLE-VCH: Weinheim, Germany, 1999; pp. 124–125. [Google Scholar]
- Vicente-García, E.; Kielland, N.; Lavilla, R. Functionalization of Heterocycles by MCRs. In Multicomponent Reactions in Organic Synthesis, 1st ed.; Zhu, J., Wang, Q., Wang, M., Eds.; Wiley-VCH: Weinheim, Germany, 2015; pp. 159–206. [Google Scholar]
- Banfi, L.; Basso, A.; Riva, R. Synthesis of Heterocycles Through Classical Ugi and Passerini Reactions Followed by Secondary Transformations Involving One or Two Additional Functional Groups. In Synthesis of Heterocycles via Multicomponent Reactions I, 1st ed.; Orru, R.V.A., Ruijter, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 23, pp. 1–8. [Google Scholar]
- Ugi, I.; Meyr, R.; Fetzer, U.; Steinsbrückner, C. Versuche mit Isonitrikn. Angew. Chem. 1959, 71, 386. [Google Scholar]
- Ugi, I.; Steinbruckner, C. Reaktion von isonitrilen mit carbonylverbindungen, aminen und stickstoffwasserstoffsäure. Chem. Ber. 1961, 94, 734–742. [Google Scholar] [CrossRef]
- Wei, C.X.; Bian, M.; Gong, G.H. Tetrazolium compounds: Synthesis and applications in medicine. Molecules 2015, 20, 5528–5553. [Google Scholar] [CrossRef] [PubMed]
- Neochoritis, C.G.; Zhao, T.; Dömling, A. Tetrazoles via multicomponent reactions. Chem. Rev. 2019, 119, 1970–2042. [Google Scholar] [CrossRef] [PubMed]
- Dheer, D.; Singh, V.; Shankar, R. Medicinal attributes of 1, 2, 3-triazoles: Current developments. Bioorg. Chem. 2017, 71, 30–54. [Google Scholar] [CrossRef] [PubMed]
- Schröder, D.C.; Kracker, O.; Fröhr, T.; Góra, G.; Jewginski, M.; Nieß, A.; Antes, I.; Latajka, R.; Marion, A.; Sewald, N. 1, 4-Disubstituted 1 H-1, 2, 3-Triazole Containing Peptidotriazolamers: A New Class of Peptidomimetics With Interesting Foldamer Properties. Front. Chem. 2019, 7, 155. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.I. One-Pot Construction of Bis-Heterocycles through Isocyanide Based Multicomponent Reactions. ChemistrySelect 2020, 5, 8040–8061. [Google Scholar] [CrossRef]
- Soural, M.; Bouillon, I.; Krchňak, V. Combinatorial libraries of bis-heterocyclic compounds with skeletal diversity. J. Comb. Chem. 2008, 10, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among US FDA approved pharmaceuticals: Miniperspective. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef] [PubMed]
- Shahrisa, A.; Esmati, S. Three novel sequential reactions for the facile synthesis of a library of bisheterocycles possessing the 3-aminoimidazo [1,2-a] pyridine core catalyzed by bismuth (III) chloride. Synlett 2013, 24, 595–602. [Google Scholar] [CrossRef]
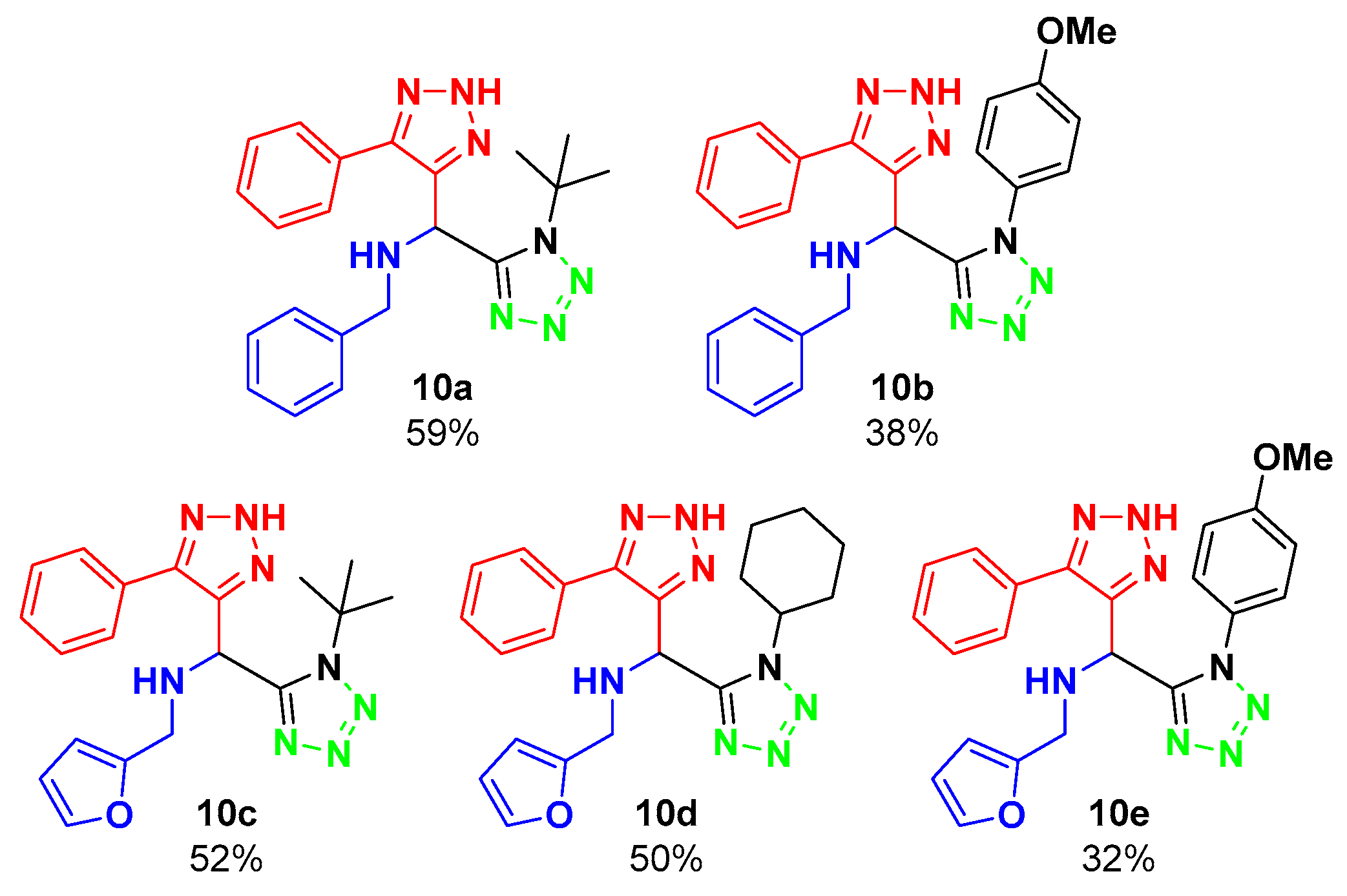
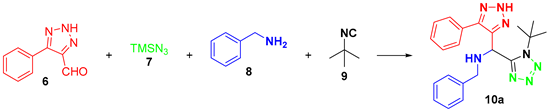 | |||
|---|---|---|---|
| Entry | Solvent | Time | Yield |
| 1 | EtOH | 24 h | 59% |
| 2 | H2O | 48 h | NR |
| 3 | - | 48 h | NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez-Lopez, F.; Zárate-Hernández, C.; Rentería-Gómez, M.A.; Gámez-Montaño, R. One-Pot Synthesis of Tetrazole–Triazole Bis-Heterocycles via Ugi–Azide Reaction. Chem. Proc. 2023, 14, 108. https://doi.org/10.3390/ecsoc-27-16060
Rodriguez-Lopez F, Zárate-Hernández C, Rentería-Gómez MA, Gámez-Montaño R. One-Pot Synthesis of Tetrazole–Triazole Bis-Heterocycles via Ugi–Azide Reaction. Chemistry Proceedings. 2023; 14(1):108. https://doi.org/10.3390/ecsoc-27-16060
Chicago/Turabian StyleRodriguez-Lopez, Fidel, Carlos Zárate-Hernández, Manuel A. Rentería-Gómez, and Rocío Gámez-Montaño. 2023. "One-Pot Synthesis of Tetrazole–Triazole Bis-Heterocycles via Ugi–Azide Reaction" Chemistry Proceedings 14, no. 1: 108. https://doi.org/10.3390/ecsoc-27-16060
APA StyleRodriguez-Lopez, F., Zárate-Hernández, C., Rentería-Gómez, M. A., & Gámez-Montaño, R. (2023). One-Pot Synthesis of Tetrazole–Triazole Bis-Heterocycles via Ugi–Azide Reaction. Chemistry Proceedings, 14(1), 108. https://doi.org/10.3390/ecsoc-27-16060






