Suzuki–Miyaura Cross-Coupling for the Synthesis of Key Intermediates of Ketoprofen and Bifonazole Analogues †
Abstract
:1. Introduction
2. Results and Discussion
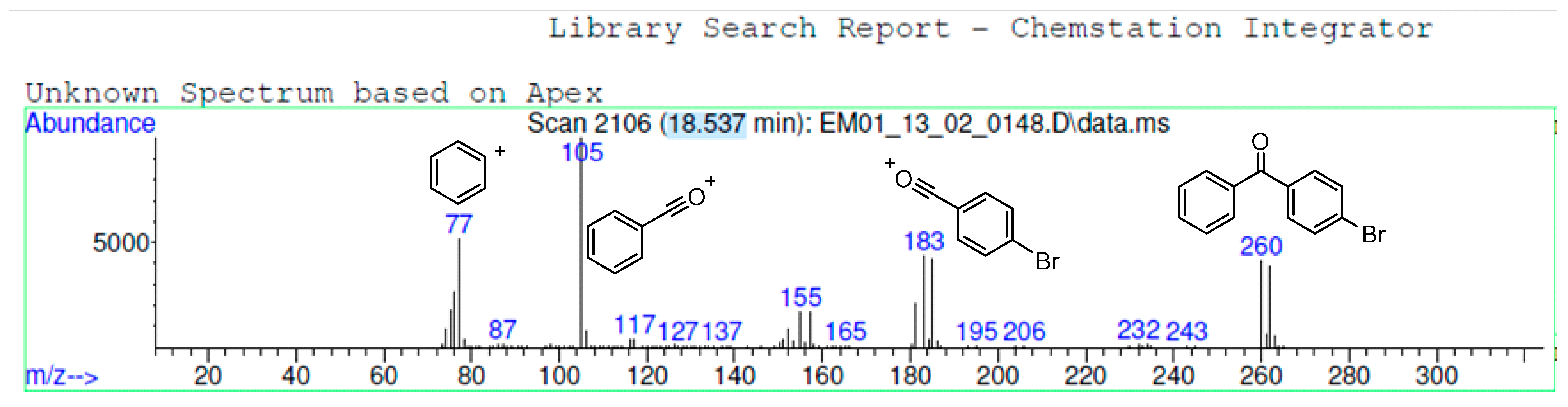
2.1. Study of the Selectivity of Suzuki Coupling: Acid Chlorides Versus Bromides
2.2. Study of the Influence of Reaction Conditions on the Suzuki Coupling between 4-bromobenzoyl Chloride and Phenylboronic Acid
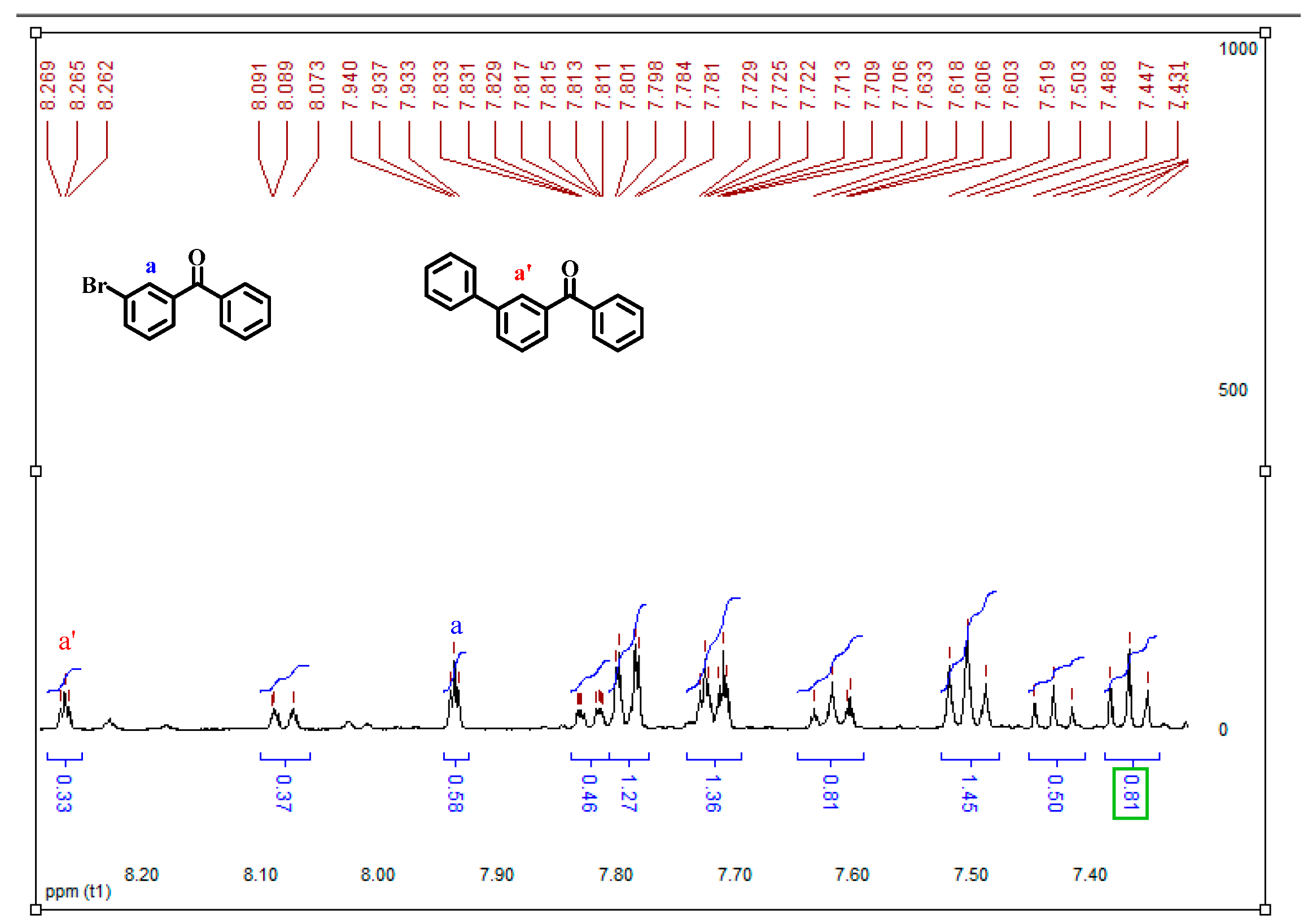
2.3. Synthesis of the Key Intermediate to Obtain Ketoprofen
3. Experimental Procedure
Typical Suzuki Couplings between Bromobenzoyl Chlorides and Phenylboronic Acids
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruzi, R.; Liu, K.; Zhu, C.; Xie, J. Upgrading ketone synthesis direct from carboxylic acids and organohalides. Nat. Commun. 2020, 11, 3312. [Google Scholar] [CrossRef] [PubMed]
- Louvis, A.R.; Silva, N.A.A.; Semaan, F.S.; Silva, F.d.C.d.; Saramago, G.; de Souza, L.C.S.V.; Ferreira, B.L.A.; Castro, H.C.; Salles, J.P.; Souza, A.L.A.; et al. Synthesis, characterization and biological activities of 3-aryl-1,4-naphthoquinones—Green palladium-catalysed Suzuki cross coupling. New J. Chem. 2016, 40, 7643. [Google Scholar] [CrossRef]
- Martins, D.d.L.; e Silva, N.A.D.A.; Ferreira, V.F.; Rangel, L.d.S.; dos Santos, J.A.A.; Faria, R.X. Molluskicidal activity of 3-aryl-2-hydroxy-1,4-naphthoquinones against Biomphalaria glabrata. Acta Trop. 2022, 231, 106414. [Google Scholar] [CrossRef] [PubMed]
- Farhang, M.; Akbarzadeh, A.R.; Rabbani, M.; Ghadiri, A.M. A retrospective-prospective review of Suzuki–Miyaura reaction: From cross-coupling reaction to pharmaceutical industry applications. Polyhedron 2022, 227, 116124. [Google Scholar] [CrossRef]
- Hooshmand, S.E.; Hiedari Bahreh Sedghi, R.; Varma, R.S. Recent advances in the Suzuki-Miyaura cross-coupling reaction using efficient catalysts in ecofriendly media. Green Chem. 2018, 21, 381–405. [Google Scholar] [CrossRef]
- Martins, D.d.L.; Alvarez, H.M.; Aguiar, L.C. Microwave-assisted Suzuki reaction catalyzed by Pd(0)-PVP nanoparticles. Tetrahedron Lett. 2010, 51, 6814. [Google Scholar] [CrossRef]
- Martins, D.L. Kumada-Corriu-Tamao couplings catalyzed by Ni and Pd as an important tool for the biaryl synthesis. Rev. Chem. 2010, 2, 231. [Google Scholar] [CrossRef]
- Koy, M.; Sandfort, F.; Tlahuext-Aca, A.; Quach, L.; Daniliuc, C.G.; Glorius, F. Palladium-Catalyzed Decarboxylative Heck-Type Coupling of Activated Aliphatic Carboxylic Acids Enabled by Visible Light. Chem. A Eur. J. 2018, 24, 4552. [Google Scholar] [CrossRef]
- Patra, T.; Maiti, D. Decarboxylation as the Key Step in C–C Bond-Forming Reactions. Chem. A Eur. J. 2017, 23, 7382. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, N.; Goossen, L.J. Decarboxylative coupling reactions: A modern strategy for C–C-bond formation. Chem. Soc. Rev. 2011, 40, 5030. [Google Scholar] [CrossRef]
- Martins, D.L.; Aguiar Lúcia, C.S.; Antunes, O.A.C. Microwave promoted Suzuki reactions between aroyl chlorides and boronic acids catalyzed by heterogeneous and homogeneous phosphine-free palladium catalysts. J. Organomet. Chem. 2011, 696, 2845. [Google Scholar] [CrossRef]
- Martins, D.d.L.; Alvarez, H.M.; Aguiar, L.C.; Antunes, O. Palladium Catalyzed Decarbonylative Mizoroki-Heck Reactions of Benzoyl Chloride and Styrene Under Microwave Irradiation. Lett. Org. Chem. 2007, 4, 253. [Google Scholar] [CrossRef]
- Ramminger, C.; Zim, D.; Lando, V.R.; Fassina, V.; Monteiro, A.L. Transition-metal catalyzed synthesis of Ketoprofen. J. Braz. Chem. Soc. 2000, 11, 105–111. [Google Scholar] [CrossRef]







| PhB(OH)2 (mmol) | Pd2dba3 | Toluene | Yield % | |
|---|---|---|---|---|
| 1 | 0.52 mmol | 1.00% | 1.0 mL | 80% |
| 2 | 0.52 mmol | 0.50% | 1.0 mL | 81% |
| 3 | 0.52 mmol | 0.25% | 1.0 mL | 86% |
| 4 | 0.50 mmol | 0.50% | 1.0 mL | 71% |
| 5 | 0.50 mmol | 0.50% | 1.0 mL | 76% |
| 6 | 0.52 mmol | 0.50% | 2.5 mL | 76% |
| 7 | 0.52 mmol | 0.25% | 2.5 mL | 67% |
| 8 | 0.52mmol | 0.10% | 2.5 mL | 70% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, N.A.d.A.e.; Martins, D.d.L. Suzuki–Miyaura Cross-Coupling for the Synthesis of Key Intermediates of Ketoprofen and Bifonazole Analogues. Chem. Proc. 2023, 14, 105. https://doi.org/10.3390/ecsoc-27-16068
Silva NAdAe, Martins DdL. Suzuki–Miyaura Cross-Coupling for the Synthesis of Key Intermediates of Ketoprofen and Bifonazole Analogues. Chemistry Proceedings. 2023; 14(1):105. https://doi.org/10.3390/ecsoc-27-16068
Chicago/Turabian StyleSilva, Nayane Abreu do Amaral e, and Daniela de Luna Martins. 2023. "Suzuki–Miyaura Cross-Coupling for the Synthesis of Key Intermediates of Ketoprofen and Bifonazole Analogues" Chemistry Proceedings 14, no. 1: 105. https://doi.org/10.3390/ecsoc-27-16068
APA StyleSilva, N. A. d. A. e., & Martins, D. d. L. (2023). Suzuki–Miyaura Cross-Coupling for the Synthesis of Key Intermediates of Ketoprofen and Bifonazole Analogues. Chemistry Proceedings, 14(1), 105. https://doi.org/10.3390/ecsoc-27-16068





