One-Pot Synthesis of Imidazo[2,1-b]thiazole via Groebke–Blackburn–Bienaymé Reaction under Free Catalyts †
Abstract
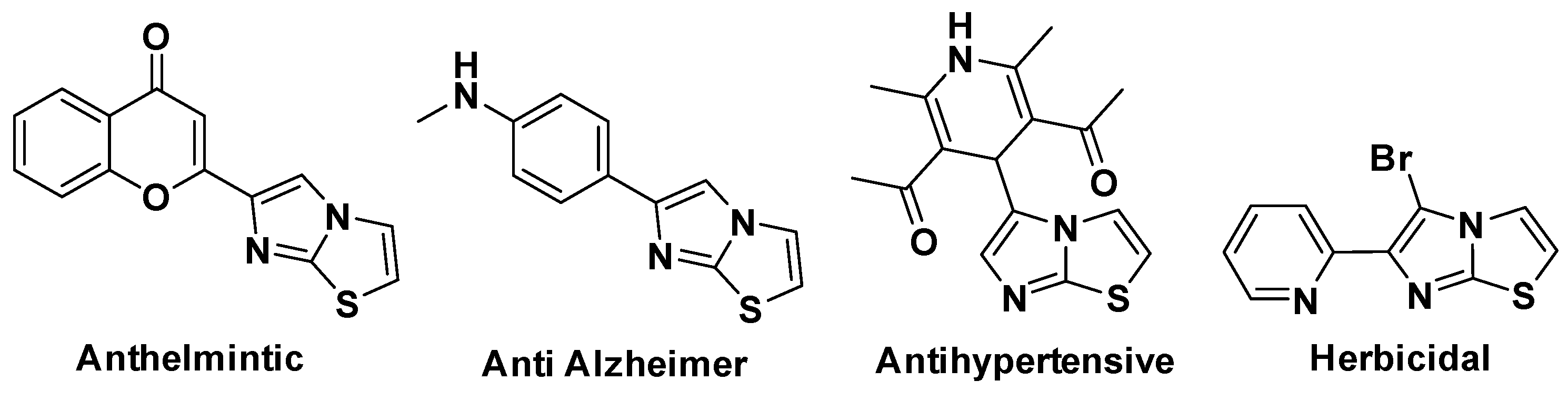
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. General Information, Instrumentation, and Chemicals
3.2. General Procedure (GP)
3.3. Spectral Data
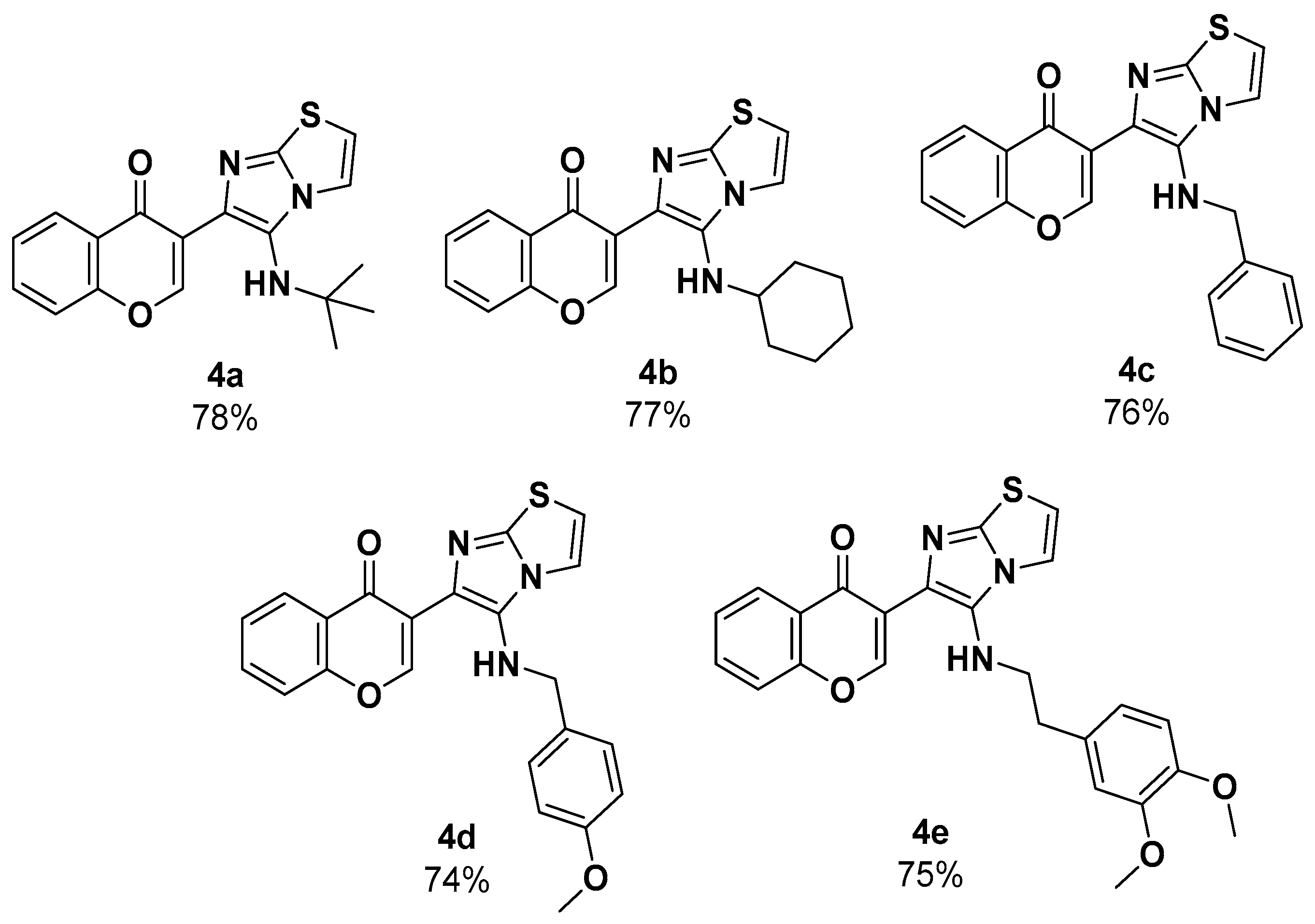
3.3.1. Synthesis and Characterization of the 3-(5-(tert-butylamino)imidazo[2,1-b]thiazol-6-yl)-4H-chromen-4-one (4a)
- According to the GP, 3-formylchromone (44.8 mg, 1 mmol), 2-aminothiazole (25.8 mg, 1 mmol), and tert-butyl isocyanide (27.9 μL, 1 mmol) were reacted together in anhydrous toluene (0.5 mL) to afford the product 4a (66 mg, 78%) as orange solid; mp = 228 °C; Rf = 0.38 (hexanes–AcOEt = 7/3; v/v). 1H NMR (500 MHz; CDCl3; TMS): δ 8.66 (s, 1H), 8.34 (d, J = 8.0 Hz, 1H), 7.73–7.69 (m, 1H), 7.54 (d, J = 8.4 Hz, 1H), 7.47 (d, J = 4.5 Hz, 1H), 7.44 (d, J = 7.9 Hz, 1H), 6.72 (d, J = 4.5 Hz, 1H), 4.90 (s, 1H), 1.05 (s, 9H); 13C NMR (126 MHz, CDCl3; TMS): δ 176.0, 156.0, 155.6, 145.6, 133.6, 130.4, 130.3, 126.4, 125.3, 124.2, 121.4, 118.3, 118.2, 111.1, 55.6, 29.7; FT-IR (ATR) Vmax/cm−1 3286 (N-H), 1629 (C=O); HRMS (ESI+): m/z calcd. for C18H18N3O2S+ 340.1114, found to be 340.1118.
3.3.2. Synthesis and Characterization of the 3-(5-(Cyclohexylamino)imidazo[2,1-b]thiazol-6-yl)-4H-chromen-4-one (4b)
- According to the GP, 3-formylchromone (44.8 mg, 1 mmol), 2-aminothiazole (25.8 mg, 1 mmol), and cyclohexyl isocyanide (31.7 µL, 1.0 mmol) were reacted together in anhydrous toluene (0.5 mL) to afford the product 4b (70 mg, 77%) as brown solid; mp = 170 °C; Rf = 0.31 (hexanes–AcOEt = 7/3; v/v). 1H NMR (500 MHz; CDCl3; TMS): δ 8.70 (s, 1H), 8.34 (d, J = 8.0 Hz, 1H), 7.70 (t, J = 8.4 Hz, 1H), 7.53 (d, J = 8.4 Hz, 1H), 7.47–7.43 (m, 1H), 7.38 (d, J = 4.5 Hz, 1H), 6.74 (d, J = 4.5 Hz, 1H), 2.76–2.69 (m, 1H), 1.87–1.80 (m, 2H), 1.70–1.64 (m, 2H), 1.55–1.50 (m, 1H), 1.18–1.07 (m, 5H); 13C NMR (126 MHz, CDCl3; TMS): δ 176.2, 156.1, 155.3, 144.9, 133.7, 132.1, 126.8, 126.5, 125.3, 124.3, 121.0, 118.3, 117.5, 111.6, 57.8, 34.1, 25.8, 25.2; FT-IR (ATR) Vmax/cm−1 3291 (N-H), 1638 (C-O); HRMS (ESI): m/z calcd. for C20H20N3O2S+ 366.1270, found to be 366.1271.
3.3.3. Synthesis and Characterization of the 3-(5-(Benzylamino)imidazo[2,1-b]thiazol-6-yl)-4H-chromen-4-one (4c)
- According to the GP, 3-formylchromone (44.8 mg, 1 mmol), 2-aminothiazole (25.8 mg, 1.0 mmol), and benzyl isocyanide (31.1 µL, 1.0 mmol) were reacted together in anhydrous toluene (0.5 mL) to afford the product 4c (71 mg, 76%) as orange solid; mp = 124 °C; Rf = 0.32 (hexanes–AcOEt = 1/3; v/v). 1H NMR (500 MHz; CDCl3; TMS): δ 8.45 (s, 1H), 8.27 (dd, J = 8.0, 1.4 Hz, 1H), 7.71–7.67 (m, 1H), 7.49 (d, J = 8.0 Hz, 1H), 7.45–7.41 (m, 1H), 7.27–7.25 (m, 1H), 7.13–7.10 (m, 2H), 7.09–7.06 (m, 3H), 6.71 (d, J = 4.5 Hz, 1H), 5.69 (s, 1H), 4.06 (s, 1H); 13C NMR (126 MHz, CDCl3; TMS): δ 176.3, 156.0, 155.1, 139.5, 133.6, 132.2, 128.5, 128.4, 127.2, 126.4, 125.3, 124.2, 118.2, 117.3, 111.8, 53.6; FT-IR (ATR) Vmax/cm−1 3282 (N-H), 1631(C-O); HRMS (ESI): m/z calcd. for C21H16N3O2S+ 374.0957, found to be 374.0963.
3.3.4. Synthesis and Characterization of the 3-(5-((4-Methoxybenzyl)amino)imidazo[2,1-b]thiazol-6-yl)-4H-chromen-4-one (4d)
- According to the GP, 3-formylchromone (44.8 mg, 1.0 mmol), 2-aminothiazole (25.8 mg, 1.0 mmol), and 4-methoxybenzyl isocyanide (37.5 mg, 1.0 mmol) were reacted together in anhydrous toluene (1.0 mL) to afford the product 4d (74 mg, 74%) as pale yellow oil; Rf = 0.11 (hexanes–AcOEt = 7/3; v/v). 1H NMR (500 MHz; CDCl3; TMS): δ 8.42 (s, 1H), 8.26 (d, J = 8.0 Hz, 1H), 7.71–7.67 (m, 1H), 7.49 (d, J = 8.5 Hz, 1H), 7.46–7.42 (m, 1H), 7.30 (d, J = 3.9 Hz, 1H), 6.95 (d, J = 8.0 Hz, 2H), 6.72 (d, J = 3.8 Hz, 1H),6.54 (d, J = 7.8 Hz, 2H), 5.51 (s, 1H), 3.97 (s, 2H), 3.61 (s, 3H); 13C NMR (126 MHz, CDCl3; TMS): δ 176.1, 158.9, 155.9, 155.1, 133.6, 132.0, 131.6, 129.9, 129.0, 127.4, 126.4, 125.3, 124.2, 118.2, 117.3, 114.3, 113.7, 111.8, 55.2, 53.1; FT-IR (ATR) Vmax/cm−1 3282 (N-H), 1638 (C=O); HRMS (ESI): m/z calcd. for C22H18N3O3S+ 404.1063, found to be 404.1083.
3.3.5. Synthesis and Characterization of the 3-(5-((3,4-Dimethoxyphenethyl)amino)imidazo[2,1-b]thiazol-6-yl)-4H-chromen-4-one (4e)
- According to the GP, 3-formylchromone (44.8 mg, 1.0 mmol), 2-aminothiazole (25.8 mg, 1.0 mmol), and 3,4-dimethoxyphenethyl isocyanide (50 mg, 1.0 mmol) were reacted together in anhydrous toluene (1.0 mL) to afford the product 4e (84 mg, 75%) as pale brown solid; mp = 152.8 °C; Rf = 0.30 (hexanes–AcOEt = 7/3; v/v). 1H NMR (500 MHz; CDCl3; TMS): δ 8.65 (s, 1H), 8.23 (d, J = 7.9 Hz, 1H), 7.74–7.69 (m, 1H), 7.53 (d, J = 8.0 Hz, 1H), 7.47–7.43 (m, 1H), 7.27 (s, 1H), 7.25–7.22 (m, 1H), 6.77–6.75 (m, 1H), 6.70–6.66 (m, 2H), 4.12 (s, 1H), 3.80 (s, 3H), 3.74 (s, 3H), 3.28–3.24 (m, 2H), 2.74–2.70 (m, 2H); 13C NMR (126 MHz, CDCl3; TMS): δ 176.0, 155.9, 155.0, 148.8, 147.4, 144.8, 133.6, 132.6, 131.7, 126.3, 125.2, 123.9, 120.6, 120.2, 118.1, 117.1, 112.1, 112.0, 111.1, 55.8, 50.0, 36.1; FT-IR (ATR) Vmax/cm−1 3319 (N-H), 1608 (C-O); HRMS (ESI): m/z calcd. for C24H22N3O4S+ 448.1325, found to be 448.1315.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hamid, A.; Philippe, R.L.; Catarina, B.; Raymond, C.; Marc, P.; Philippe, M.L.; Christian, B.; Gayral, P. Imidazo[2,1-b]thiazoles: Analogues du lévamisole. Eur. J. Med. Chem. 1987, 22, 463. [Google Scholar]
- Yousefi, B.H.; Manook, M.; Drzezga, A.; Reutern, B.V.; Schwaiger, M.; Wester, H.J.; Henriksen, G. Synthesis and evaluation of 11C-labeled imidazo[2,1-b]benzothiazoles (IBTs) as PET tracers for imaging β-amyloid plaques in Alzheimer’s disease. J. Med. Chem. 2011, 54, 949. [Google Scholar] [CrossRef] [PubMed]
- Budriesi, R.; Loan, P.; Locatelli, A.; Cosconati, S.; Leoni, A.; Ugenti, M.P.; Andreani, A.; Toro, R.D.; Bedini, A.; Spampinato, S.; et al. Imidazo[2,1-b]thiazole system: A scaffold endowing dihydropyridines with selective cardiodepressant activity. J. Med. Chem. 2008, 51, 1592. [Google Scholar] [CrossRef] [PubMed]
- Andreani, A.; Rambaldi, M.; Leoni, A.; Locatelli, A.; Andreani, F.; Gehret, J.-C. Synthesis of imidazo[2,1-b]thiazoles as herbicides. Pharm. Acta Helv. 1996, 71, 247. [Google Scholar] [CrossRef]
- Cesurl, Z.; Gunerl, H.; Otuk, G. Synthesis and antimycobacterial activity of new imidazo[2,1-b]thiazole derivatives. Eur. J. Med. Chem. 1994, 29, 981. [Google Scholar] [CrossRef]
- Bakherad, M.; Keivanloo, A.; Bahramian, B.; Kamali, T.A. Synthesis of novel 6-(substituted benzyl) imidazo[2,1-b][1,3]thiazole catalyzed by polystyrene-supported palladium (ii) ethylenediamine complex. J. Braz. Chem. Soc. 2009, 20, 907. [Google Scholar] [CrossRef]
- Dzhavakhishvili, S.G.; Borisov, A.V.; Nikitchenko, V.M.; Kovalenko, S.N. Features of the reaction of unsymmetrical 2-mercapto-imidazoles with aromatic and aliphatic ketones. Chem. Heterocycl. Compd. 2007, 43, 98–105. [Google Scholar] [CrossRef]
- Boltjes, A.; Dömling, A. The Groebke-Blackburn-Bienaymé Reaction. Eur. J. Org. Chem. 2019, 42, 7007–7049. [Google Scholar] [CrossRef] [PubMed]
- Manvar, P.; Shaikh, F.; Kakadiya, R.; Mchariya, K.; Khunt, R.; Pandey, B.; Shah, A. Synthesis of novel imidazo[1,2-a]pyridine-4-hydroxy-2H-coumarins by Groebke–Blackburn–Bienaymé multicomponent reaction as potential NS5B inhibitors. Tetrahedron 2016, 72, 1293. [Google Scholar] [CrossRef]
- Keri, R.S.; Budagumpi, S.; Pai, R.K.; Balakrishna, R.G. Chromones as a privileged scaffold in drug discovery: A review. Eur. J. Med. Chem. 2014, 78, 340–374. [Google Scholar] [CrossRef] [PubMed]
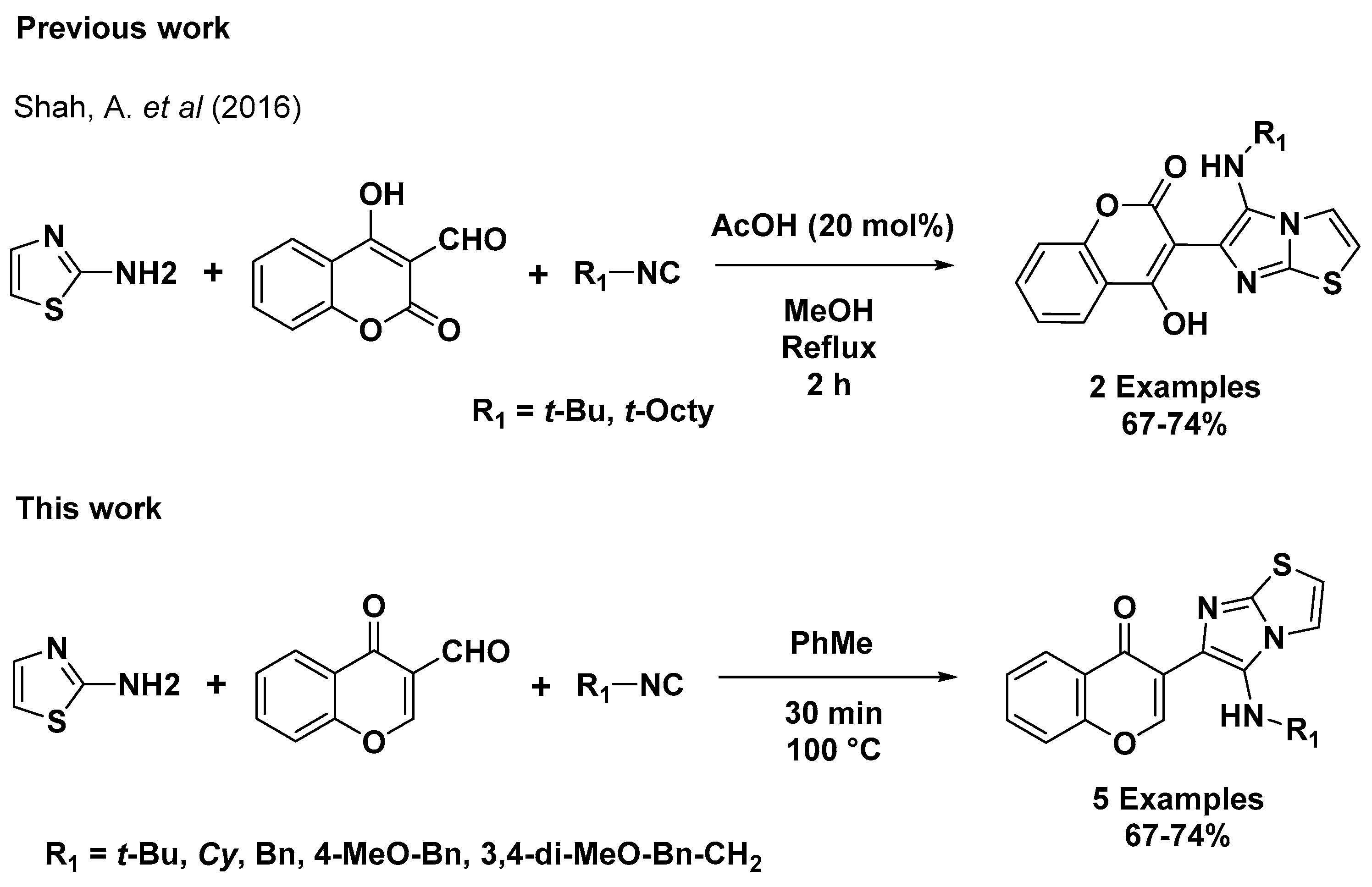
| Entry | Solvent | Time (min) | Temp °C | Yield |
|---|---|---|---|---|
| 1 | MeOH | 60 | 85 | 33 |
| 2 | MeCN | 60 | 85 | 40 |
| 3 | PhMe | 60 | 85 | 68 |
| 4 | PhMe | 30 | 100 | 78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calderón-Rangel, D.; Pérez, K.A.G.; Díaz, A.C.; Gámez-Montaño, R. One-Pot Synthesis of Imidazo[2,1-b]thiazole via Groebke–Blackburn–Bienaymé Reaction under Free Catalyts. Chem. Proc. 2023, 14, 103. https://doi.org/10.3390/ecsoc-27-16095
Calderón-Rangel D, Pérez KAG, Díaz AC, Gámez-Montaño R. One-Pot Synthesis of Imidazo[2,1-b]thiazole via Groebke–Blackburn–Bienaymé Reaction under Free Catalyts. Chemistry Proceedings. 2023; 14(1):103. https://doi.org/10.3390/ecsoc-27-16095
Chicago/Turabian StyleCalderón-Rangel, David, Karla A. González Pérez, Alejandro Corona Díaz, and Rocío Gámez-Montaño. 2023. "One-Pot Synthesis of Imidazo[2,1-b]thiazole via Groebke–Blackburn–Bienaymé Reaction under Free Catalyts" Chemistry Proceedings 14, no. 1: 103. https://doi.org/10.3390/ecsoc-27-16095
APA StyleCalderón-Rangel, D., Pérez, K. A. G., Díaz, A. C., & Gámez-Montaño, R. (2023). One-Pot Synthesis of Imidazo[2,1-b]thiazole via Groebke–Blackburn–Bienaymé Reaction under Free Catalyts. Chemistry Proceedings, 14(1), 103. https://doi.org/10.3390/ecsoc-27-16095






