Abstract
The application of isocyanide-based multicomponent reactions (IMCRs) for triterpenoid functionalization has been little reported. Triterpenoids and their derivatives are an important class of natural products of interest in medicinal chemistry due to their potential applications as antibacterial, antifungal, and cytotoxic agents. Herein, we describe the use of ethanol as a solvent in the Passerini reaction for the functionalization of masticadienonic acid isolated from fruits and peduncles of P. mexicana. A small series of α-acyloxycarboxamides was synthesized with moderate to good overall yields of 33 to 57%, evaluating and extending the scope of the aldehyde component.
1. Introduction
Triterpenoids are a large class of plant-derived natural products, with an inherent structural diversity [1]. They are widely distributed in the plant kingdom, especially in higher plants [2]. Approximately 55,000 compounds belonging to this group have been identified; however, very few have been investigated for therapeutic applications [3]. The prevention and treatment of cancer is the most studied property of triterpenoids. Other important aspects include their antimicrobial, antiviral, antifungal, antiparasitic, anti-inflammatory, etc., properties [4,5,6,7,8,9].
Multicomponent reactions (MCRs) are synthetic procedures where three or more substances react to form a product which contains all or most of the atoms from the starting materials [10]. In these procedures, a complex molecule is assembled in a one pot chemical step, providing a huge chemical diversity [11].
Among the diverse class of MCRs, those involving isocyanide reagents were some of the first to be discovered. Mario Passerini reported in 1921 the first isocyanide-based multicomponent reaction (IMCR), employing aryl isocyanides, ketones, and carboxylic acids to obtain α-acyloxycarboxamides [12]. Almost 40 years later, Ivar Ugi presented his four-component reactions, in which an isocyanide, a carbonyl compound, an amine, and an isocyanide react to yield bis-amides [13].
In the field of combinatorial chemistry, the IMCRs are an important and versatile tool which have several advantages, such as atom economy, convergent design, ease of performance, and the generation of molecular diversity [14]. On the other hand, IMCR products can be versatile synthetic platforms for further structural diversification [15].
In recent years, IMCRs have been applied to the rapid and efficient functionalization of natural products, especially steroids, peptides, and glycosides [16]. However, there are only a few reports on the use of triterpenoids as components in IMCRs. Regarding the Passerini reaction, there are two reports where triterpenoids were either naturally functionalized or their derivatives were used as components (Figure 1) [17,18]. Triterpenoids and their amide derivatives have been extensively studied over the last few decades as an alternative for cancer treatment, due to their important cytotoxic properties [19].
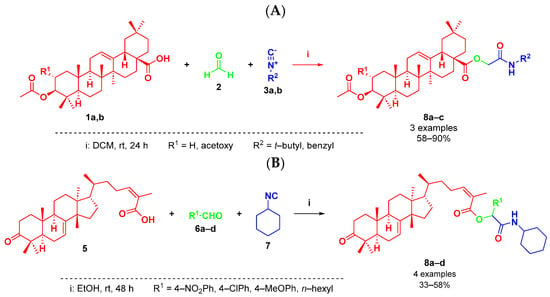
Figure 1.
(A) Previous work by Wiemann, J., et al. (2018) [18]. (B) This work.
2. Results and Discussion
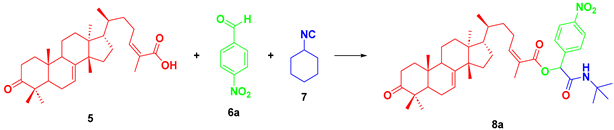
In the present work, we report the synthesis of a series of α-acyloxycarboxamides employing a plant-derived triterpenoid as component in the Passerini three-component reactions. Masticadienonic acid (5) isolated from hexane extracts of fruits and peduncles of dried P. mexicana, along with 4-nitrobenzaldehyde (6a) and cyclohexyl isocyanide (7), was used as a component for reaction optimization to synthesize target molecule 8a. The performed experiments are depicted in Table 1.

Table 1.
Screening conditions for the synthesis of target molecule 16a.
In previous reports from our research group, dichloromethane was used as a solvent for the Passerini reaction; however, to improve the greenness of our procedures, we also utilized alternative solvents. There are some reports of the use of polar protic solvents for this methodology, for example methanol; however, this experiment led to a longer reaction time and a low yield of 30%. The starting material was not fully consumed, which might be a consequence of the poor solubility of compound 5. Another experiment using ethanol resulted in better yields (57%) since 5 is fairly soluble in this solvent. Finally, an experiment using water did not yield the desired product under the evaluated conditions.
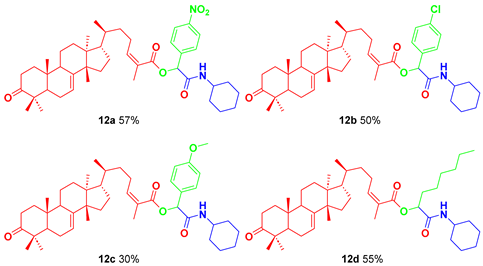
Using the best conditions found in these experiments, a small library of α-acyloxycarboxamides was synthesized, employing aromatic aldehydes with electron-donor and electron-withdrawing groups, as well as an aliphatic aldehyde.
3. Experimental Section
3.1. General Information, Chemicals, and Instrumentation
Bruker Avance III spectrometers (500 and 125 MHz, respectively) were used for 1H and 13C NMR spectra acquisition. Deuterated chloroform (CDCl3) was used as the solvent for NMR experiments. Chemical shifts (δ) are given in ppm relative to tetramethylsilane (TMS). Coupling constants are reported in Hertz (Hz). The multiplicities of the signals are described using standard abbreviations: singlet (s), doublet (d), triplet (t), quartet (q), and multiplet (m). NMR spectra were analyzed using MestReNova software version 12.0.0-20080. Reaction progress was monitored via thin-layer chromatography (TLC) on pre-coated silica gel F254 aluminum sheets. The spots were visualized under UV light at 254 nm. Column chromatography was performed using silica gel (230–400 mesh) as stationary phase. Mixtures of hexanes and ethyl acetate were used as mobile phase for column chromatography and in TLC for reaction progress monitoring and measuring retention factors (Rf). All reagents were purchased from Sigma-Aldrich and were used without further purification. Chemical names and drawings were obtained using the ChemDraw 22.2.0.3300 software package.
3.2. General Procedure
Masticadienonic acid (5, 1.0 equiv.), aldehyde 6a–d (1.0 equiv.), and cyclohexyl isocyanide (7, 1.0 equiv.) were dissolved in ethanol (0.5 M) and placed in a sealed vial with a magnetic stir bar. The mixture was stirred at room temperature for 48 h. Then, solvent was removed under reduced pressure, and the crude reaction mixture was purified via column chromatography, using silica gel as stationary phase and a mixtures of ethyl acetate in hexanes, to yield the corresponding α-acyloxycarboxamides 8a–d.
3.3. Spectral Data
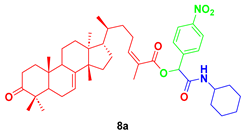
2-(cyclohexylamino)-1-(4-nitrophenyl)-2-oxoethyl-3-oxotirucalla-7,24Z-dien-26-oate (8a). White solid; Rf = 0.3 (20% ethyl acetate in hexanes): 1H NMR (500 MHz, CDCl3, 25 °C, TMS): δ 8.23 (m, 2H), 7.63 (m, 2H), 6.14 (s, 1H), 6.10 (td, J = 7.6, 1.6 Hz, 1H), 6.00 (s, 1H), 5.30 (dd, J = 6.1, 3.1 Hz, 1H), 3.84 (dtt, J = 10.4, 7.8, 4.0 Hz, 1H), 2.76 (td, J = 14.5, 5.5 Hz, 1H), 2.59 (m, 1H), 2.45 (m, 1H), 2.28 (dt, J = 14.1, 3.8, 1H), 2.25 (m, 1H), 2.10 (m, 2H), 2.07 (d, J = 1.5 Hz, 3H), 1.99 (m, 2H), 1.98 (m, 1H), 198 (m, 4H), 1.81 (m, 2H), 1.73 (t, J = 8.7 Hz, 1H), 1.69 (m, 2H), 1.64 (m, 1H), 1.56 (m, 2H), 1.53 (m, 2H), 1.49 (m, 1H), 1.48 (m, 2H), 1.48 (m, 1H), 1.40 (m, 1H), 1.28 (m, 1H), 1.18 (m, 4H) 1.14 (m, 1H), 1.12 (s, 3H), 1.05 (s, 3H), 1.01 (s, 3H), 1.01 (s, 3H), 0.89 (d, J = 6.2 Hz, 3H), 0.81 (s, 3H); 13C NMR (126 MHz, CDCl3) δ 216.8, 166.3, 165.0, 148.9, 146.6, 145.88, 143.2, 127.9, 125.2, 123.7, 117.8, 74.2, 52.9, 52.3, 51.1, 48.4, 47.8, 47.7, 43.5, 38.5, 36.0, 35.6, 35.0, 34.9, 34.0, 33.63, 32.9, 28.2, 27.4, 26.9, 25.4, 24.6, 24.5, 24.3, 21.9, 21.6, 20.6, 18.2, 18.2, 12.7.
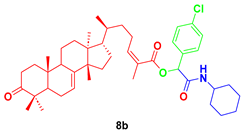
2-(cyclohexylamino)-1-(4-chlorophenyl)-2-oxoethyl-3-oxotirucalla-7,24Z-dien-26-oate (8b). Yellow oil; Rf = 0.31 (20% ethyl acetate in hexanes): 1H NMR (500 MHz, CDCl3, 25 °C, TMS): δ 7.40 (m, 2H), 7.35 (m, 2H), 6.04 (d, J = 8.2 Hz, 1H), 6.00 (dt, J = 7.6, 1.6 Hz, 1H), 5.98 (d, J = 2.6 Hz, 1H), 5.31 (dd, J = 6.0, 3.2 Hz, 1H), 3.85 (dtt, J = 10.5, 7.8, 4.1 Hz, 1H), 2.77 (td, J = 14.5, 5.4 Hz, 1H), 2.58 (m, 1H), 2.45 (m, 1H), 2.29 (dt, J = 14.1, 3.7, 1H), 2.24 (m, 1H), 2.10 (m, 2H), 1.99 (m, 2H), 1.98 (m, 1H), 1.98 (d, J = 1.6 Hz, 3H), 1.93 (m, 4H), 1.82 (m, 1H), 1.73 (t, J = 8.7 Hz, 1H), 1.69 (m, 2H) 1.65 (m, 1H), 1.56 (m, 2H), 1.53 (m, 2H), 1.51 (m, 1H), 1.48 (m, 2H), 1.47 (m, 1H), 1.40 (m, 1H), 1.36 (s, 9H), 1.29 (m, 1H), 1.18 (m, 4H), 1.14 (m, 1H), 1.12 (s, 3H), 1.06 (s, 3H), 1.01 (s, 3H), 1.00 (s, 3H), 0.89 (d, J = 6.2 Hz, 3H), 0.80 (s, 3H); 13C NMR (126 MHz, CDCl3) δ 216.8, 167.3, 165.7, 146.6, 145.9, 135.0, 134.5, 129.1, 128.8, 125.2, 117.9, 73.9, 52.9, 52.3, 51.2, 48.5, 47.9, 47.7, 43.5, 38.5, 36.1, 35.7, 35.0, 34.9, 34.1, 33.6, 33.0, 28.3, 27.4, 27.0, 25.4, 24.7, 24.5, 24.4, 22.0, 21.6, 20.7, 18.2, 18.2, 12.8.
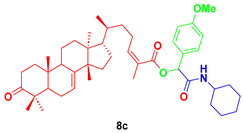
2-(cyclohexylamino)-1-(4-methoxyphenyl)-2-oxoethyl-3-oxotirucalla-7,24Z-dien-26-oate (8c). Yellow oil; Rf = 0.38 (20% ethyl acetate in hexanes): 1H NMR (400 MHz, CDCl3, 25 °C, TMS): δ 7.34 (m, 2H), 6.87 (m, 2H), 6.09 (dt, J = 7.5, 1.2 Hz, 1H), 6.02 (bs, 1H), 5.98 (d, J = 2.9 Hz, 1H), 5.31 (dd, J = 6.1, 3.4 Hz, 1H), 3.86 (dtt, J = 10.5, 7.7, 4.0 Hz, 1H), 3.80 (s, 3H), 2.74 (td, J = 14.5, 5.4 Hz, 1H), 2.59 (m, 1H), 2.44 (m, 1H), 2.28 (dt, J = 14.1, 3.8, 1H), 2.25 (m, 1H), 2.11 (m, 2H), 1.98 (m, 1H), 1.99 (m, 1H), 1.97 (d, J = 1.5 Hz, 3H), 193 (m, 4H), 1.81 (m, 1H), 1.73 (t, J = 8.7 Hz, 1H), 169 (m, 2H), 1.65 (m, 1H), 1.56 (m, 2H), 1.55 (m, 2H), 1.49 (m, 1H), 1.48 (m, 2H), 1.48 (m, 1H), 1.41 (m, 1H), 1.36 (s, 9H), 1.28 (m, 1H), 1.18 (M, 4H), 1.15 (m, 1H), 1.12 (s, 3H), 1.05 (s, 3H), 1.01 (s, 3H), 0.99 (s, 3H), 0.89 (d, J = 6.2 Hz, 3H), 0.81 (s, 3H); 13C NMR (126 MHz, CDCl3) δ 216.8, 166.3, 165.7, 159.8, 146.8, 145.8, 128.8, 128.4, 125.3, 117.7, 75.0, 52.9, 52.3, 51.2, 48.6, 47.8, 47.7, 43.5, 38.5, 36.0, 35.6, 35.0, 34.9, 34.0, 33.6, 32.9, 28.2, 27.4, 26.8, 25.4, 24.4, 24.4, 24.2, 21.9, 21.6, 20.6, 18.3, 18.2, 12.8.
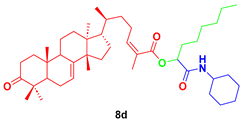
1-(cyclohexylamino)-1-oxooctan-2-yl-3-oxotirucalla-7,24Z-dien-26-oate (8d). Yellow oil; Rf = 0.55 (20% ethyl acetate in hexanes): 1H NMR (400 MHz, CDCl3, 25 °C, TMS): δ 7.39 (m, 2H), 7.35 (m, 2H), 6.06 (bs, 1H), 6.03 (dt, J = 7.6, 1.6 Hz, 1H), 5.97 (d, J = 2.6 Hz, 1H), 5.30 (dd, J = 6.0, 3.2 Hz, 1H), 3.83 (dtt, J = 10.4, 7.9, 4.1 Hz, 1H), 2.77 (td, J = 14.5, 5.4 Hz, 1H), 2.59 (m, 1H), 2.45 (m, 1H), 2.28 (dt, J = 14.1, 3.8, 1H), 2.25 (m, 1H), 2.10 (m, 2H), 1.99 (m, 2H), 1.97 (m, 1H), 1.98 (d, J = 1.6 Hz, 3H), 193 (m, 4H), 1.82 (m, 1H), 1.76 (m, 2H), 1.73 (t, J = 8.7 Hz, 1H), 169 (m, 2H), 1.64 (m, 1H), 1.56 (m, 2H), 1.53 (m, 2H), 1.49 (m, 1H), 1.48 (m, 2H), 1.48 (m, 1H), 1.47 (m, 2H), 1.40 (m, 1H), 1.36 (s, 9H), 1.31 (m, 2H), 1.29 (m, 2H), 1.28 (m, 1H), 1.26 (m, 2H), 1.18 (m, 4H), 1.16 (m, 1H), 1.11 (s, 3H), 1.05 (s, 3H), 1.01 (s, 3H), 1.00 (s, 3H), 0.89 (d, J = 6.2 Hz, 3H), 0.88 (t, J = 6.7 Hz, 3H) 0.81 (s, 3H); 13C NMR (126 MHz, CDCl3) δ 216.9, 166.3, 165.8, 146.7, 145.9, 125.3, 117.8, 74.0, 52.9, 52.3, 51.2, 48.5, 47.8, 47.8, 43.4, 38.5, 36.1, 35.5, 35.0, 34.9, 34.0, 33.6, 32.9, 31.7, 31.6, 28.9, 28.2, 27.4, 26.9, 25.4, 24.6, 24.4, 24.5, 24.3, 22.5, 21.9, 21.6, 20.6, 18.4, 18.3, 14.0, 12.8.
4. Conclusions
Herein we developed an efficient and versatile IMCR of Passerini for the functionalization of natural products like triterpenoids. It is important to highlight that alternative solvents can be used for improving the greenness of the Passerini reaction. Aliphatic and deactivated aromatic aldehydes led to the best results in our study, as was expected. Finally, it should be highlighted that the source of the used triterpenoid was sustainable and was obtained without causing harm to the plant and its environment.
Author Contributions
Conceptualization, R.G.-M. and H.A.G.-G.; methodology, F.R.-L. and E.G.R.-G.; software, F.R.-L.; validation, R.G.-M., F.R.-L. and R.G.-M.; formal analysis, R.G.-M.; investigation, F.R.-L. and R.G.-M.; resources, R.G.-M.; data curation, F.R.-L.; writing—original draft preparation, F.R.-L.; writing—review and editing, R.G.-M.; visualization, R.G.-M.; supervision, R.G.-M. and H.A.G.-G.; project administration, R.G.-M.; funding acquisition, R.G.-M. and H.A.G.-G. All authors have read and agreed to the published version of the manuscript.
Funding
F.R.-L. is grateful to CONACYT-Mexico for a scholarship (764724). R.G.-M. is grateful for financial support from UG CIIC 005/2022, 132/2023, and CONACYT (CB-2016-285622). H.A.G.-G. is grateful for financial support from CIC-UMSNH (6790306) and CONACYT-Mexico (grant no. A1-S-47352).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets presented in this article are not readily available because the data are part of an ongoing study. Requests to access the datasets should be directed to R.G.-M.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Springob, K.; Kutchan, T.M. Introduction to the different classes of natural products. In Plant-Derived Natural Products; Osbourn, A., Lanzotti, V., Eds.; Springer: New York, NY, USA, 2009; pp. 3–50. [Google Scholar]
- Talapatra, S.K.; Talapatra, B. Introduction: Enzymes. Cofactors/Coenzymes. Primary and Secondary Metabolites. Natural Products and their Functions. Plant Chemical Ecology. Biosynthesis. Metabolic Pathways. In Chemistry of Plant Natural Products; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–22. [Google Scholar]
- Brahmkshatriya, P.P.; Brahmkshatriya, P.S. Terpenes: Chemistry, Biological Role, and Therapeutic Applications. In Natural Products; Ramawat, K., Mérillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 2665–2691. [Google Scholar]
- Rascón-Valenzuela, L.A.; Torres-Moreno, H.; Velázquez-Contreras, C.; Garibay-Escobar, A.; Robles-Zepeda, R.E. Triterpenoids: Synthesis, Uses in Cancer Treatment and Other Biological Activities. In Advances in Medicine and Biology; Berhardt, L.V., Ed.; Nova Science Publishers: New York, NY, USA, 2017; Volume 106, pp. 139–182. [Google Scholar]
- Chudzik, M.; Korzonek-Szlacheta, I.; Król, W. Triterpenes as Potentially Cytotoxic Compounds. Molecules 2015, 20, 1610–1625. [Google Scholar] [CrossRef] [PubMed]
- Morais, T.; da Costa-Silva, T.; Tempone, A.; Borborema, S.; Scotti, M.; de Sousa, R.; Araujo, A.; de Oliveira, A.; de Morais, S.; Sartorelli, P.; et al. Antiparasitic Activity of Natural and Semi-Synthetic Tirucallane Triterpenoids from Schinus terebinthifolius (Anacardiaceae): Structure/Activity Relationships. Molecules 2014, 19, 5761–5776. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Monroy, M.; Jacobo-Herrera, N.; Zentella-Dehesa, A.; Hernández-Téllez, B.; Martínez-Vázquez, M. Masticadienonic and 3α-OH Masticadienoic Acids Induce Apoptosis and Inhibit Cell Proliferation and Tumor Growth in Prostate Cancer Xenografts in Vivo. Molecules 2017, 22, 1479. [Google Scholar] [CrossRef] [PubMed]
- Oviedo-Chavez, I.; Ramirez-Apan, T.; Martinez-Vazquez, M. Cytotoxic Activity and Effect on Nitric Oxide Production of Tirucallane-Type Triterpenes. J. Pharm. Pharmacol. 2005, 57, 1087–1091. [Google Scholar] [CrossRef] [PubMed]
- Dzubak, P.; Hajduch, M.; Vydra, D.; Hustova, A.; Kvasnica, M.; Biedermann, D.; Markova, L.; Urban, M.; Sarek, J. Pharmacological Activities of Natural Triterpenoids and Their Therapeutic Implications. Nat. Prod. Rep. 2006, 23, 394–411. [Google Scholar] [CrossRef] [PubMed]
- Tietze, L.F.F.; Brasche, G.; Gericke, K.M. Multicomponent reactions. In Domino Reactions in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Dömling, A.E.; AlQahtani, A.D. General Introduction to MCRs: Past, Present, and Future. In Multicomponent Reactions in Organic Synthesis; Zhu, J., Wang, Q., Wang, M., Eds.; Wiley-VCH: Weinheim, Germany, 2015. [Google Scholar]
- Wahby, Y.; Abdel-Hamid, H.; Salah Ayoup, M. Two decades of recent advances of Passerini reactions: Synthetic and potential pharmaceutical applications. New J. Chem. 2022, 46, 1445. [Google Scholar] [CrossRef]
- Fouad, M.A.; Abdel-Hamid, H.; Ayoup, M.S. Two Decades of Recent Advances of Ugi Reactions: Synthetic and Pharmaceutical Applications. RSC Adv. 2020, 10, 42644–42681. [Google Scholar] [CrossRef] [PubMed]
- Rudick, J.G.G.; Shaabani, S.; Dömling, A. Isocyanide-based multicomponent reactions. Front. Chem. 2020, 7, 918. [Google Scholar] [CrossRef] [PubMed]
- Răzvan, C.; Ruijter, C.; Orru, R.V. Multicomponent Reactions: Advanced Tools for Sustainable Organic Synthesis. Green Chem. 2014, 16, 2958–2975. [Google Scholar]
- Reguera, L.; Attorresi, C.I.; Ramírez, J.A.; Rivera, D.G. Steroid Diversification by Multicomponent Reactions. Beilstein J. Org. Chem. 2019, 15, 1236–1256. [Google Scholar] [CrossRef] [PubMed]
- Gurrapu, S.; Walsh, W.J.; Brooks, J.M.; Jonnalagadda, S.C.; Mereddy, V.R. Synthesis of Lupane Triterpenoid Derivatives. Nat. Prod. Indian J. 2012, 8, 115–120. [Google Scholar]
- Wiemann, J.; Heller, L.; Csuk, R. An Access to a Library of Novel Triterpene Derivatives with a Promising Pharmacological Potential by Ugi and Passerini Multicomponent Reactions. Eur. J. Med. Chem. 2018, 150, 176–194. [Google Scholar] [CrossRef] [PubMed]
- Sommerwerk, S.; Heller, L.; Kuhfs, J.; Csuk, R. Selective Killing of Cancer Cells with Triterpenoic Acid Amides—The Substantial Role of an Aromatic Moiety Alignment. Eur. J. Med. Chem. 2016, 122, 452–464. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).