The Influence of Calcium–Sodium Ion Exchange in the Rheometry of Sodium Alginate-Based Hydrogel †
Abstract
:1. Introduction
2. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Veronica, N.; Heng, P.W.S.; Liew, C.V. Alginate-based matrix tablets for drug delivery. Expert Opin. Drug Deliv. 2023, 20, 115–130. [Google Scholar] [CrossRef]
- Morozkina, S.; Strekalovskaya, U.; Vanina, A.; Snetkov, P.; Krasichkov, A.; Polyakova, V.; Uspenskaya, M. The Fabrication of Alginate-Carboxymethyl Cellulose-Based Composites and Drug Release Profiles. Polymers 2022, 14, 3604. [Google Scholar] [CrossRef] [PubMed]
- Noroozi, R.; Arif, Z.U.; Taghvaei, H.; Khalid, M.Y.; Sahbafar, H.; Hadi, A.; Sadeghianmaryan, A.; Chen, X.B. 3D and 4D Bioprinting Technologies: A Game Changer for the Biomedical Sector? Ann. Biomed. Eng. 2023, 51, 1683–1712. [Google Scholar] [CrossRef] [PubMed]
- Urena, M.; Fournier, P.; Phung, T.T.T.; Lagorce, A.; Karbowiak, T. Potential of polysaccharides for food packaging applications. Part 2/2: An experimental review of the effect of aging conditions on the functional properties of polysaccharide films. Food Hydrocoll. 2023, 144, 108954. [Google Scholar] [CrossRef]
- Vanitha, N.; Shanmugapriya, C.; Selvasekarapandian, S.; Vignesh, N.M.; Hazaana, S.A.; Naachiyar, R.M.; Devi, S.K. Synthesis of biopolymer electrolyte using sodium alginate with ammonium perchlorate (NH4ClO4) for the application of electrochemical devices. Ionics 2023, 29, 4037–4054. [Google Scholar] [CrossRef]
- Berg, J.; Seiffert, S. Composite hydrogels based on calcium alginate and polyethyleneimine for wastewater treatment. J. Polym. Sci. 2023, 61, 2203–2222. [Google Scholar] [CrossRef]
- Charisis, A.; Kalogianni, E.P. Alginate-Chitosan Microgel Particles, Water-Oil Interfacial Layers, and Emulsion Stabilization. Colloids Interfaces 2023, 7, 48. [Google Scholar] [CrossRef]
- Du, M.T.; Huang, L.L.; Peng, M.K.; Hu, F.Y.; Gao, Q.; Chen, Y.S.; Liu, P. Preparation of vancomycin-loaded alginate hydrogel coating on magnesium alloy with enhanced anticorrosion and antibacterial properties. Thin Solid Film. 2020, 693, 137679. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, W.T.; Chen, S.Y.; Liang, H.S.; Li, J.; Li, Y.; Li, B. Pickering emulsions stabilized by homogenized ball-milled eggshell particles in combination with sodium alginate. Int. J. Biol. Macromol. 2023, 229, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.J.; Jin, W.J.; Liu, S.F.; Cheng, X.W.; Guan, J.P.; Yang, X.H. Flame retardancy and combustion performance of polysaccharide fabrics: A comparison on cotton and calcium alginate fabrics. Polym. Test. 2023, 124, 108099. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Lu, Y. Sodium Alginate-Based Functional Materials toward Sustainable Applications: Water Treatment and Energy Storage. Ind. Eng. Chem. Res. 2023, 62, 11279–11304. [Google Scholar] [CrossRef]
- Nan, D.Y.; Xu, S.D.; Chen, L.; Lu, Z.H.; Zhang, D.; Wei, T.; Chen, J.Q.; Li, Z. Stepwise induced passivation film formation on the surface of spherical hard carbons enabling ultra-stable potassium ions storage. J. Power Sources 2023, 579, 233299. [Google Scholar] [CrossRef]
- Shin, S.; Hyun, J. Matrix-Assisted In Situ Polymerization of a 3D Conductive Hydrogel Structure. Acs Appl. Mater. Interfaces 2022, 14, 52516–52523. [Google Scholar] [CrossRef]
- Mallakpour, S.; Azadi, E.; Hussain, C.M. State-of-the-art of 3D printing technology of alginate-based hydrogels—An emerging technique for industrial applications. Adv. Colloid Interface Sci. 2021, 293, 102436. [Google Scholar] [CrossRef] [PubMed]
- Ramdhan, T.; Ching, S.H.; Prakash, S.; Bhandari, B. Time dependent gelling properties of cuboid alginate gels made by external gelation method: Effects of alginate-CaCl2 solution ratios and pH. Food Hydrocoll. 2019, 90, 232–240. [Google Scholar] [CrossRef]
- Cook, W. Alginate dental impression materials: Chemistry, structure, and properties. J. Biomed. Mater. Res. 1986, 20, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.C.F.; Petronilio, J.A.; Maneschy, C.E.D.; Cruz, D. The Carreau-Yasuda fluids: A skin friction equation for turbulent flow in pipes and Kolmogorov dissipative scales. J. Braz. Soc. Mech. Sci. Eng. 2007, 29, 162–167. [Google Scholar] [CrossRef]

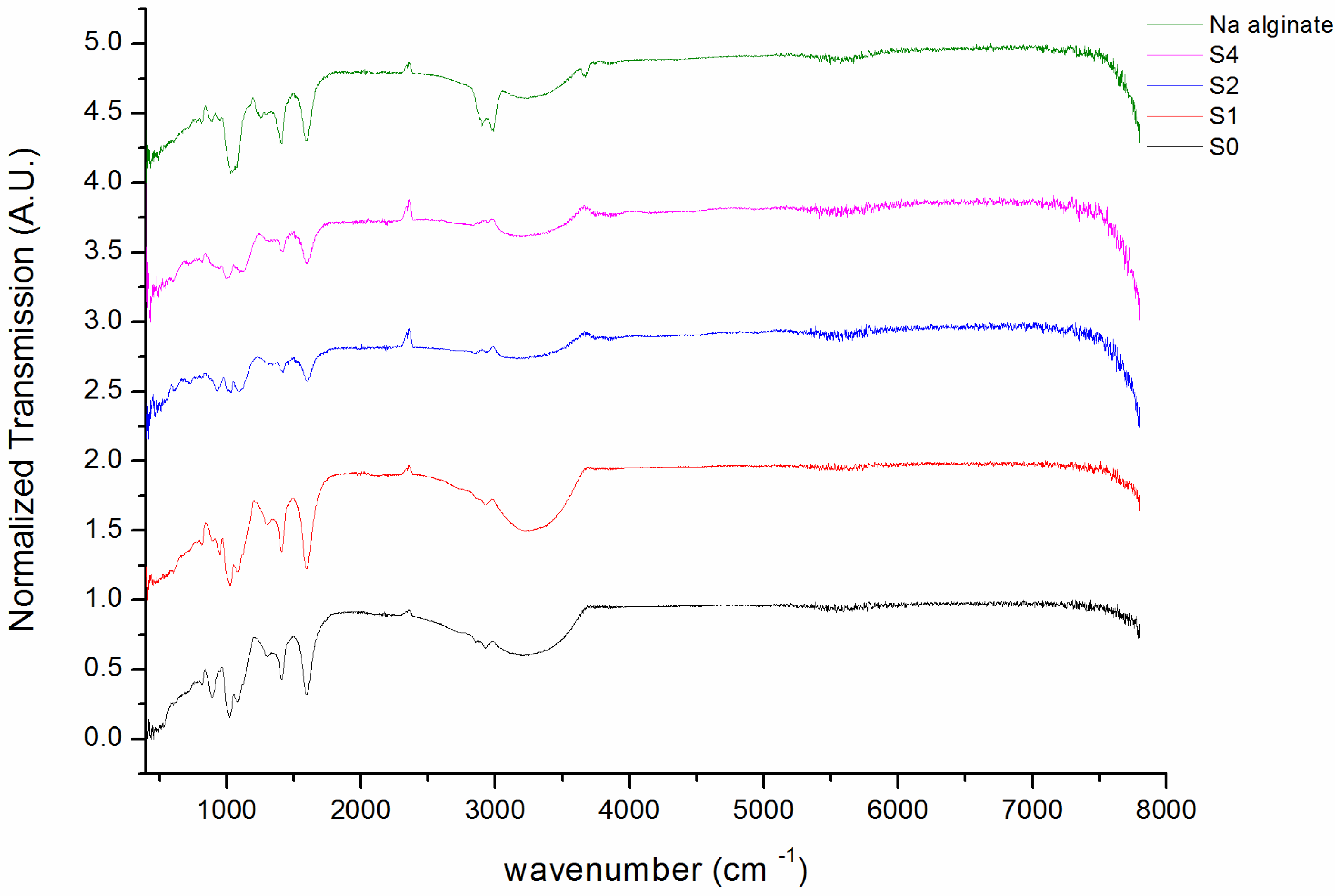
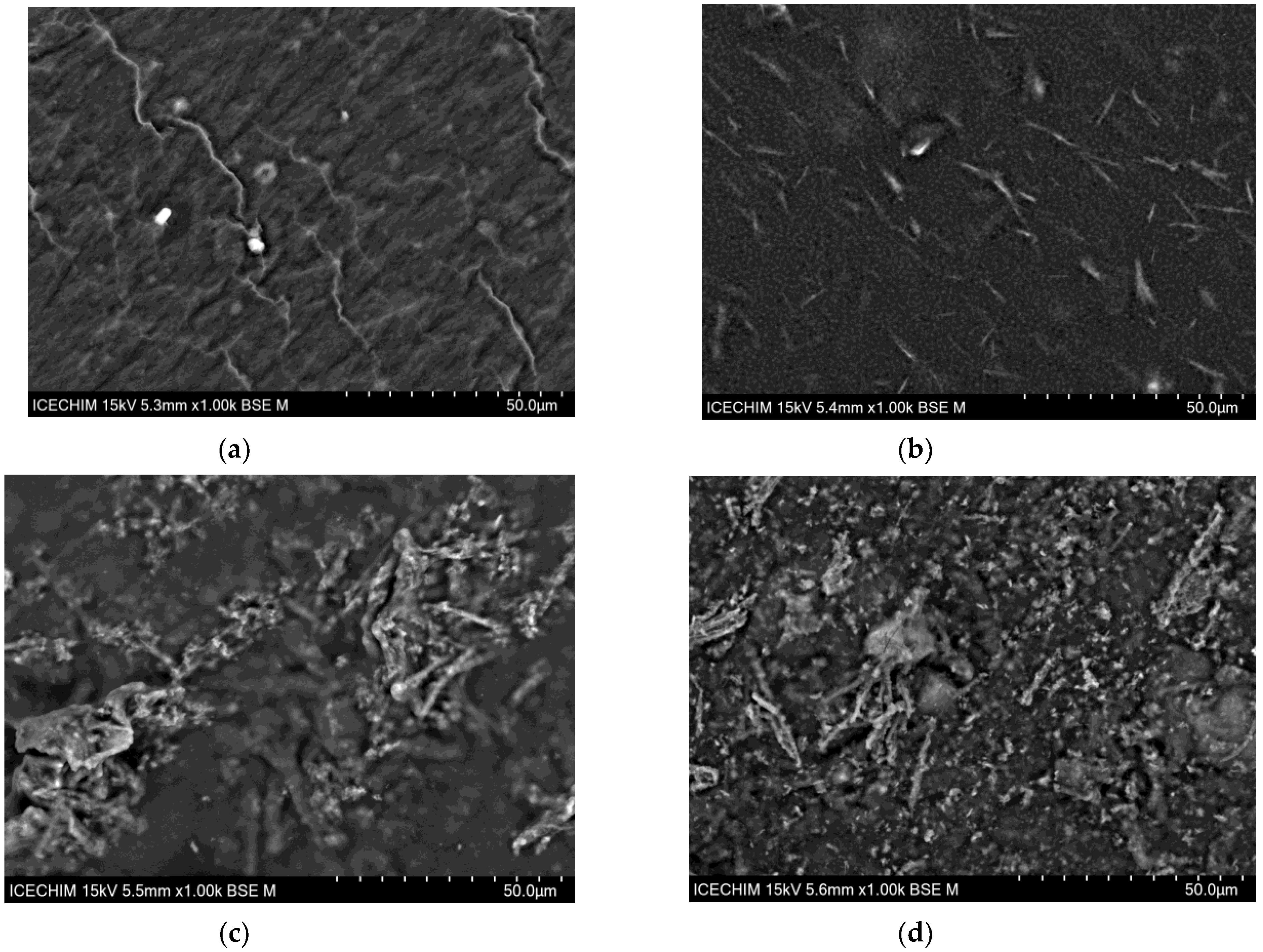
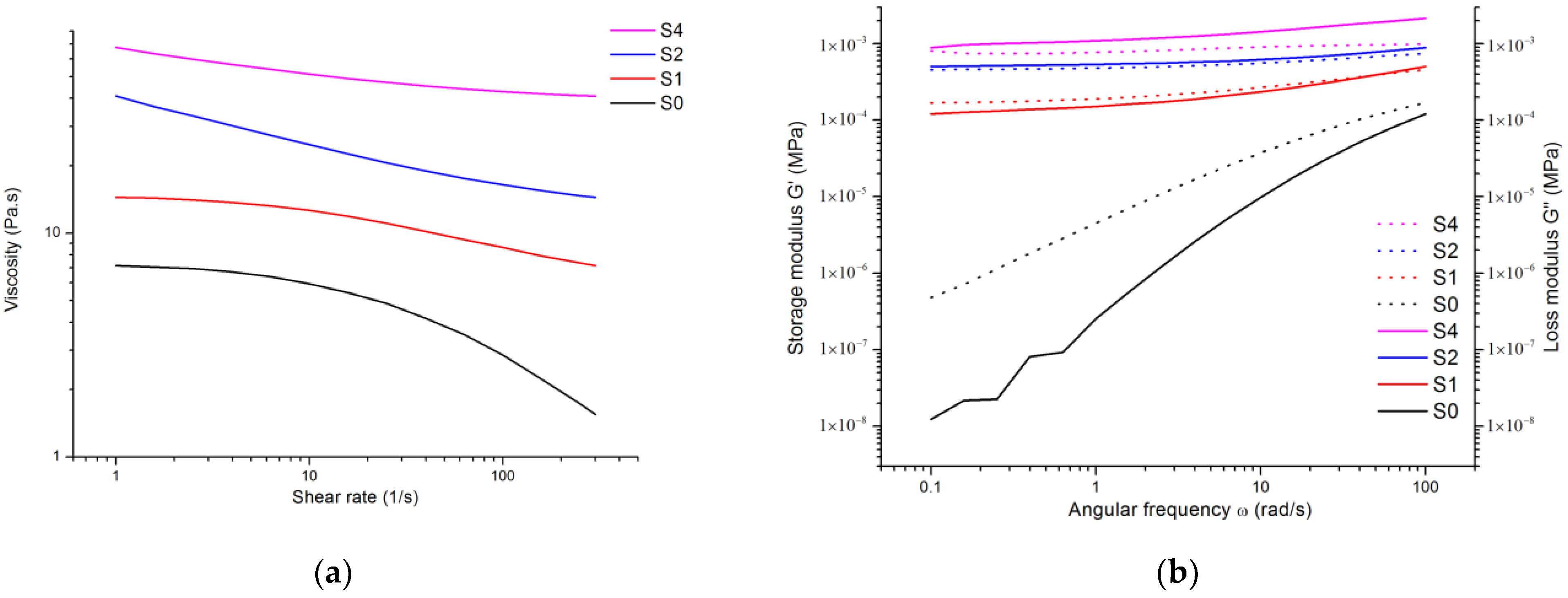
| Sample | H2O | Sodium Alginate | Tetrasodium Pyrophosphate | Calcium Sulfate | Zero-Rate Viscosity |
|---|---|---|---|---|---|
| S0 | 100 g | 3 g | 1 g | 0 g | 5.06309 Pa·s |
| S1 | 100 g | 3 g | 1 g | 0.1689 g | 7.07305 Pa·s |
| S2 | 100 g | 3 g | 1 g | 0.3378 g | 224.198 Pa·s |
| S4 | 100 g | 3 g | 1 g | 0.6756 g | 666.185 Pa·s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghiurea, M.; Tritean, N.; Dima, Ş.-O.; Trică, B.; Hosu, I.; Oancea, F. The Influence of Calcium–Sodium Ion Exchange in the Rheometry of Sodium Alginate-Based Hydrogel. Chem. Proc. 2023, 13, 13. https://doi.org/10.3390/chemproc2023013013
Ghiurea M, Tritean N, Dima Ş-O, Trică B, Hosu I, Oancea F. The Influence of Calcium–Sodium Ion Exchange in the Rheometry of Sodium Alginate-Based Hydrogel. Chemistry Proceedings. 2023; 13(1):13. https://doi.org/10.3390/chemproc2023013013
Chicago/Turabian StyleGhiurea, Marius, Naomi Tritean, Ştefan-Ovidiu Dima, Bogdan Trică, Ioana Hosu, and Florin Oancea. 2023. "The Influence of Calcium–Sodium Ion Exchange in the Rheometry of Sodium Alginate-Based Hydrogel" Chemistry Proceedings 13, no. 1: 13. https://doi.org/10.3390/chemproc2023013013
APA StyleGhiurea, M., Tritean, N., Dima, Ş.-O., Trică, B., Hosu, I., & Oancea, F. (2023). The Influence of Calcium–Sodium Ion Exchange in the Rheometry of Sodium Alginate-Based Hydrogel. Chemistry Proceedings, 13(1), 13. https://doi.org/10.3390/chemproc2023013013









