Abstract
This work investigates the effect of a sequential extraction process, a combination of ultrasound-assisted extraction and microwave-assisted extraction, on the antioxidant activities of pectin extracted from grape pomace. Our results demonstrated the enhancement of antioxidant activity due to the extraction conditions which reduce pectin hydrolysis. Higher antioxidant activity promotes the innovative application of the pectin extracted from grape marc for seed treatment as a film-forming ingredient with a biostimulant function.
1. Introduction
The grape marc is a co-product of the winery industry and consists of grape seed, skin, lignocellulosic cell walls, and stalks [1]. The valorization of this co-product increases winery sustainability and profitability [2,3]. The most efficient approach is an integrated cascading approach following the pyramid value, i.e., the recovery of value-added products such as antioxidant polyphenols [4], including resveratrol [5] and pectic substances [6]. The extraction of pectic substances weakens the lignocellulose matrix and increases the yield of hydroxycinnamic acid bound to hemicellulose or of the flavonoids trapped inside the crushed grape cell walls [7].
Romania ranks fifth in the European Union per surface of vineyards, with more than 180,000 ha [8]. Approximately 25% of the annual harvest from wineries is represented by secondary by-products from this industry—especially grape marc [9].
To intensify the process of extracting pectin and polyphenols from grape marc, we developed a sequential extraction process, successively using two extraction methods: ultrasound-assisted extraction and microwave-assisted extraction.
Microwaves are a form of non-ionizing electromagnetic radiation that works at a frequency between 300 MHz and 300 GHz. These waves are found in the electromagnetic spectrum between infrared rays and X-rays. The direct action of this radiation on matter is to transform electromagnetic energy into thermal energy. Microwaves consist of two oscillating perpendicular fields, the magnetic and the electric, responsible for heating. Microwave-assisted extraction (MAE) depends on heating the solvent in association with the sample. In addition, the process is governed by two phenomena: dipole rotation and ion conduction. The rotation of the dipole means the realignment of the dipoles of the molecule with the rapid change in the electric field. This mechanism indicates that only dielectric materials or polar solvents are heated in the microwave field. On the other hand, ionic conduction represents the transfer of ions caused by the changing electric field. Subsequently, the migration generates friction (the resistance offered by the liquid phase), responsible for heating the solution [10]. Dry plant material can be used because plant cells have a degree of moisture susceptible to microwave heating [11]. Water evaporation produces high pressure on the cell wall, which will eventually rupture, increasing the recovery yields of phytoconstituents in the solvent [12]. This technique heats the matrix externally and internally without creating a thermal gradient, thus resulting in efficient functional extraction of the target active compounds [13].
Ultrasound-assisted extraction (AUE) is based on the cavitation phenomenon in which wave propagation and extractant mass transfer are enhanced by high shear forces. Vortex acceleration and internal diffusions are caused by inter-particle collisions, high velocity, macro-turbulence, and disruption occurring in microporous particles due to the implosion of cavitation bubbles.
In addition, cavitation close to the surface of the solid phase transmits a rapid flow of liquid, resulting in particle damage, erosion, and flaking at the surface of the solid. This effect of exposing new surfaces further increases mass transfer [14]. Due to the large contact surface between the liquid and solid phases (due to the small size of the particles), EAU is significantly faster than classical techniques [15]. The acoustic cavitation of high-power ultrasound (18–40 kHz frequency) disrupts the cell wall, facilitating the accessibility of the cell contents to the solvent, thus improving mass transfer [16].
We evaluated the effect of the sequential process, wherein microwave and ultrasound are successively applied, on the antioxidant activity of the pectin separated by ethanol precipitation from polyphenols. Our results demonstrate that, under our experimental conditions, the application of the sequential process of ultrasound–microwave leads to enhancement of the antioxidant activity of the pectin extracted from grape marc, compared with conventionally extracted pectin, in hot pure water acidified with citric acid.
2. Materials and Methods
2.1. Materials
Citric acid 99% was supplied by Chimopar (Bucharest, Romania), ethyl alcohol 96% and methyl alcohol p.a. were provided by Carl Roth Chemie (Karlsruhe, Germany), and 2,2-Diphenyl-1-picrylhydrazy (DPPH) was purchased from Sigma-Aldrich (Merck Group, Darmstadt, Germany). Grape marc (produced from Vitis vinifera, cv. Fetească Neagră) was collected from a Valea Calugărească (Romania) vineyard.
2.2. Extraction of Pectin from Grape Marc
The sequential extraction (SE) process used in this work involves the successive application of microwave-assisted extraction and ultrasound-assisted extraction. The working conditions and parameters of the extraction of pectin (and polyphenols) from grape marc, using ultrapure (Milli-Q) water acidified with citric acid as the solvent, are presented in Table 1.

Table 1.
Extraction conditions of pectin from grape marc.
The ultrasonic reactor used for pectin and polyphenol extraction was a piece of ultrasonic equipment (Model BR-30C1, Banry, Lishui, Zhejiang, China). For the microwave-assisted extraction of pectin and polyphenols from grape marc, we used a Minilabotron 2000 microwave reactor (Sairem, Décines-Charpieu, France).
The conventional extraction (CE) of pectin and polyphenols from grape marc was carried out with ultrapure (Milli-Q) water acidified with citric acid. The grape marc—water ratio was 1:10, and the mixture was maintained at 90 °C for 3 h in a water heating bath, HBR 4 control (IKA, Staufen, Germany).
After the extractions, sequential or conventional, the pectin was separated, purified, and dried by the following protocol: centrifugation for 15 min at 6000× g, (Universal 320 R, Hettich, Tuttlingen, Germany); precipitation of the supernatant with ethyl alcohol 96% (ratio supernatant:ethyl alcohol 1:1); filtration of the vaccum filter; purification of the pectin by repeated washing with ethyl alcohol 96%; and drying at 50 °C for 24 h in a drying oven (ED36, Binder, Tuttlingen, Germany). The dried pectin samples were analyzed for their antioxidant activities.
2.3. The Antioxidant Activity of the Pectin
The antioxidant activity of the pectin was assayed by the DPPH radical scavenging method, adapted for pectin samples [17]. The DPPH radical scavenging activity of the grape marc pectin solutions with different concentrations (0.1 mg/mL–10 mg/mL) was correlated with the concentrations for the determination of the IC50 values for the two types of analyzed grape marc pectin, SE- and CE-extracted. The analyses were carried out for three different batches of grape marc, from the same grape cultivar (Fetească Neagră). The assays for DPPH scavenging activity were carried out in triplicate for each pectin batch.
2.4. Statistical Analysis
The data were analyzed by one-way ANOVA by using IBM® SPSS® Statistics, version 26 (IBM SPSS Corp., Armonk, NY, USA). The results are expressed as the mean values ± standard deviation (SD).
3. Results and Discussion
3.1. Experimental Set-Up and Calibration
The ultrasound reactor used for the first step of the sequential extraction is presented in Figure 1.

Figure 1.
Ultrasound reactor used for the first step of pectin extraction: (a) the ultrasound equipment with the sonotrode and (b) the control panel.
The calibration curve of the ultrasound-assisted extraction process is presented in Figure 2.
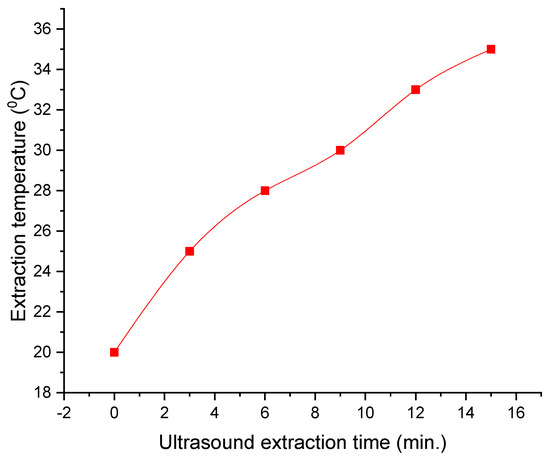
Figure 2.
Calibration curve of the ultrasound-assisted extraction process.
The experimental set-up of the microwave reactor used for the second extraction step of the sequential extraction is shown in Figure 3. The extraction vessel is also equipped with a reflux condenser to capture volatiles and maintain the extraction balance. The installation can work at volumes between 100 mL and a max. of 5 L and is equipped with a cooling system for the extraction vessel to maintain controlled temperatures. Therefore, thermal degradation of thermolabile compounds, including pectin, does not occur.
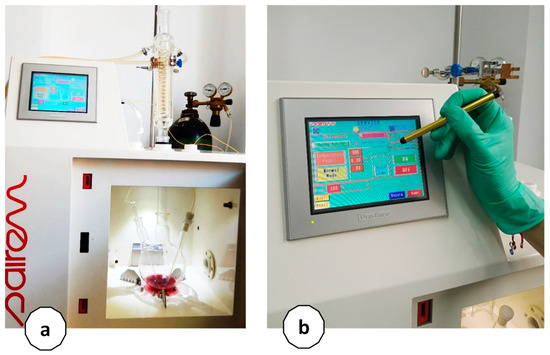
Figure 3.
Experimental set-up for microwave-assisted extraction: (a) the extraction vessel is equipped with a reflux condenser to capture volatiles and maintain the extraction balance and (b) the digital control system.
The Minilabotron 2000 reactor is equipped with a digital control system for the extraction parameters with a touch screen, a thermocouple, an IR sensor, and optical fiber for monitoring the temperature inside and outside the extraction vessel, an adapter for magnetic and/or mechanical stirring of the extraction mixture, a flow meter, and a secured outlet for introducing inert gas into the extraction vessel. The microwave source is a continuous wave (CW) magnetron of 2 kW and a frequency of 2450 MHz. The microwave power is adjustable from 0 to 2 kW with an increment of 10 W. The control interface monitors the reflected power, incident power, extraction time, and temperature. The maximum volume of extraction/batch is 3000 mL.
The variation of the extraction parameters during microwave heating is illustrated in Figure 4. The temperature was maintained between 32 and 75 °C.
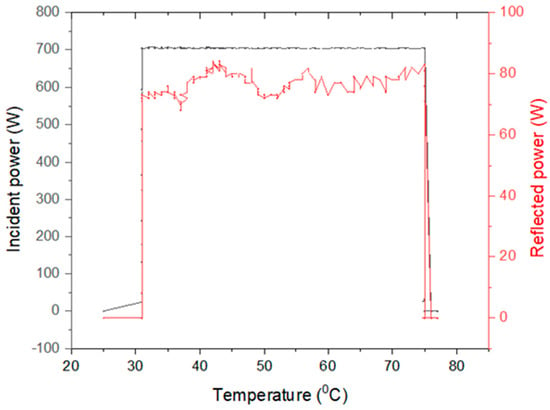
Figure 4.
Variation of extraction parameters during the microwave heating process: incident power/reflected power versus extraction temperature.
3.2. The Antioxidant Activity of the Extracted Pectin
The IC50 for pectin extracted by the sequential process developed in this work is 2.8 ± 0.1 mg/mL and is significantly lower than the IC50 determined for conventionally extracted pectin, which is 4.1 ± 0.4 mg/mL. The antioxidant activity of the sequentially extracted pectin is higher than that of the CE pectin because a smaller concentration determines 50% scavenging of the DPPH radical.
The enhancement of antioxidant activity is probably related to the lower degradative extraction condition during the sequential extraction, leading to higher quality pectin. The ultrasounds induce cavitation bubbles, breaking the squeezed lignocellulosic matrix wherein pectin is trapped, promoting water penetration and mass transfer [18]. Microwave extraction improves pectin quality due to shorter and more targeted delivery of the thermal energy intended to intensify water diffusion and pectin extraction [19]. The targeted delivery of thermal energy is demonstrated by the temperature level, which did not exceed 75 °C.
The antioxidant activity of the pectin is related to its higher molecular weight, higher number of free -OH and -COOH groups, and higher branching [20]. Less degradative extraction conditions should lead to higher quality pectin, i.e., higher molecular weight, higher branching, and more free -OH and -COOH groups.
Such high quality for the extracted pectin should also determine better characteristics for innovative application of the pectin extracted from grape marc for seed treatment as a film-forming ingredient with biostimulant functions. Non-microbial biostimulants regulate seed germination and seedling development through several mechanisms, including antioxidant defense and activation of the plant defense systems [21]. Film-forming pectin for seed coating, with higher antioxidant activity, could contribute to antioxidant defense. The formation of oligosaccharides, acting as damage-associated molecular patterns (DAMPs), determines the activation of the plant defense system [22,23]. Such activation has recently been demonstrated to promote seed germination and early seedling growth [24].
4. Conclusions
The sequential process for pectin extraction from grape marc, developed in this work, produces higher quality pectin, more suitable for application as a bioactive seed coating. The set-up of the microwave-assisted extraction system with a cooling system and a reflux condenser to capture volatiles and maintain the extraction balance reduces the thermal energy transferred to the grape marc-acidified water mixture. The sequential extraction system is less energy-intensive than conventional extraction, which involves maintaining the grape marc and the acidulated water at 96 °C for 3 h. Scale-up of the sequential process should determine process optimization, with further carbon footprint reduction.
Author Contributions
Conceptualization, M.P. and F.O.; methodology, M.P. and F.O.; validation, S.K. and F.O.; formal analysis, M.P.; investigation, M.P. and S.K.; resources, S.K. and F.O.; data curation, F.O.; writing—original draft preparation, M.P.; writing—review and editing, F.O.; visualization, S.K.; supervision, F.O.; project administration, M.P. and F.O.; funding acquisition, S.K. and F.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Cohesion Funds of the European Union, project POC-A1-A1.2.3-G-2015-P_40_352-SECVENT, Sequential processes to close bioeconomy side stream and innovative bioproducts resulted from these, contract 81/2016, SMIS 105684, subsidiary projects 1817/2020 Pectan.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analysis, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
References
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Muhlack, R.A.; Potumarthi, R.; Jeffery, D.W. Sustainable wineries through waste valorisation: A review of grape marc utilisation for value-added products. Waste Manag. 2018, 72, 99–118. [Google Scholar] [CrossRef]
- Sirohi, R.; Tarafdar, A.; Singh, S.; Negi, T.; Gaur, V.K.; Gnansounou, E.; Bharathiraja, B. Green processing and biotechnological potential of grape pomace: Current trends and opportunities for sustainable biorefinery. Bioresour. Technol. 2020, 314, 123771. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, B.; Yadav, V.; Yadav, A.; Rahman, M.U.; Yuan, W.Z.; Li, Z.; Wang, X. Integrated biorefinery approach to valorize winery waste: A review from waste to energy perspectives. Sci. Total Environ. 2020, 719, 137315. [Google Scholar] [CrossRef]
- Rodrigues, R.P.; Gando-Ferreira, L.M.; Quina, M.J. Increasing Value of Winery Residues through Integrated Biorefinery Processes: A Review. Molecules 2022, 27, 4709. [Google Scholar] [CrossRef] [PubMed]
- Spinei, M.; Oroian, M. The Potential of Grape Pomace Varieties as a Dietary Source of Pectic Substances. Foods 2021, 10, 867. [Google Scholar] [CrossRef]
- Coelho, M.; Pereira, R.; Rodrigues, A.; Teixeira, J.; Pintado, M. The use of emergent technologies to extract added value compounds from grape by-products. Trends Food Sci. Technol. 2020, 106, 182–197. [Google Scholar] [CrossRef]
- Chiurciu, I.-A.; Zaharia, I.; Soare, E. Production of wine grapes and cultural traditions related to vine in Romania. Sci. Pap. Ser. Manag. Econ. Eng. Agric. Rural Dev. 2020, 20, 133–143. [Google Scholar]
- Fuciu, M. New Marketing Tendencies in the Romanian Wine Industry. Stud. Bus. Econ. 2020, 15, 31–39. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef]
- Liazid, A.; Schwarz, M.; Varela, R.M.; Palma, M.; Guillén, D.A.; Brigui, J.; Macías, F.A.; Barroso, C.G. Evaluation of various extraction techniques for obtaining bioactive extracts from pine seeds. Food Bioprod. Process. 2010, 88, 247–252. [Google Scholar] [CrossRef]
- Cavalloro, V.; Martino, E.; Linciano, P.; Collina, S. Microwave-Assisted Solid Extraction from Natural Matrices. In Microwave Heating—Electromagnetic Fields Causing Thermal and Non-Thermal Effects; Churyumov, G.I., Ed.; IntechOpen: Rijeka, Croatia, 2021; Chapter 2. [Google Scholar]
- Li, Y.; Radoiu, M.; Fabiano-Tixier, A.-S.; Chemat, F. From Laboratory to Industry: Scale-Up, Quality, and Safety Consideration for Microwave-Assisted Extraction. In Microwave-Assisted Extraction for Bioactive Compounds: Theory and Practice; Chemat, F., Cravotto, G., Eds.; Springer: Boston, MA, USA, 2013; pp. 207–229. [Google Scholar]
- Savun-Hekimoğlu, B. A Review on Sonochemistry and Its Environmental Applications. Acoustics 2020, 2, 766–775. [Google Scholar] [CrossRef]
- Mason, T.M.; Vinatoru, M. Power ultrasound in food technology. In Sonochemistry; Mason, T.M., Ed.; De Gruyter: Berlin, Germany; Boston, MA, USA, 2023; pp. 161–212. [Google Scholar]
- Hujjatul Islam, M.; Paul, M.T.Y.; Burheim, O.S.; Pollet, B.G. Recent developments in the sonoelectrochemical synthesis of nanomaterials. Ultrason. Sonochem. 2019, 59, 104711. [Google Scholar] [CrossRef] [PubMed]
- Calvete-Torre, I.; Muñoz-Almagro, N.; Pacheco, M.T.; Antón, M.J.; Dapena, E.; Ruiz, L.; Margolles, A.; Villamiel, M.; Moreno, F.J. Apple pomaces derived from mono-varietal Asturian ciders production are potential source of pectins with appealing functional properties. Carbohydr. Polym. 2021, 264, 117980. [Google Scholar] [CrossRef] [PubMed]
- Marić, M.; Grassino, A.N.; Zhu, Z.; Barba, F.J.; Brnčić, M.; Rimac Brnčić, S. An overview of the traditional and innovative approaches for pectin extraction from plant food wastes and by-products: Ultrasound-, microwaves-, and enzyme-assisted extraction. Trends Food Sci. Technol. 2018, 76, 28–37. [Google Scholar] [CrossRef]
- Adetunji, L.R.; Adekunle, A.; Orsat, V.; Raghavan, V. Advances in the pectin production process using novel extraction techniques: A review. Food Hydrocoll. 2017, 62, 239–250. [Google Scholar] [CrossRef]
- Megías-Pérez, R.; Ferreira-Lazarte, A.; Villamiel, M. Valorization of Grape Pomace as a Renewable Source of Techno-Functional and Antioxidant Pectins. Antioxidants 2023, 12, 957. [Google Scholar] [CrossRef]
- Gupta, S.; Doležal, K.; Kulkarni, M.G.; Balázs, E.; Van Staden, J. Role of non-microbial biostimulants in regulation of seed germination and seedling establishment. Plant Growth Regul. 2022, 97, 271–313. [Google Scholar] [CrossRef]
- Ferrari, S.; Savatin, D.V.; Sicilia, F.; Gramegna, G.; Cervone, F.; Lorenzo, G.D. Oligogalacturonides: Plant damage-associated molecular patterns and regulators of growth and development. Front. Plant Sci. 2013, 4, 49. [Google Scholar] [CrossRef]
- Benedetti, M.; Pontiggia, D.; Raggi, S.; Cheng, Z.; Scaloni, F.; Ferrari, S.; Ausubel, F.M.; Cervone, F.; De Lorenzo, G. Plant immunity triggered by engineered in vivo release of oligogalacturonides, damage-associated molecular patterns. Proc. Natl. Acad. Sci. USA 2015, 112, 5533–5538. [Google Scholar] [CrossRef]
- Udchumpisai, W.; Uttapap, D.; Wandee, Y.; Kotatha, D.; Rungsardthong, V. Promoting effect of pectic-oligosaccharides produced from pomelo peel on rice seed germination and early seedling growth. J. Plant Growth Regul. 2023, 42, 2176–2188. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).