The Effect of Microwave and Ultrasound Process Intensification on the Antioxidant Activity of Pectin Extracted from Grape Marc †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Pectin from Grape Marc
2.3. The Antioxidant Activity of the Pectin
2.4. Statistical Analysis
3. Results and Discussion
3.1. Experimental Set-Up and Calibration
3.2. The Antioxidant Activity of the Extracted Pectin
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Muhlack, R.A.; Potumarthi, R.; Jeffery, D.W. Sustainable wineries through waste valorisation: A review of grape marc utilisation for value-added products. Waste Manag. 2018, 72, 99–118. [Google Scholar] [CrossRef]
- Sirohi, R.; Tarafdar, A.; Singh, S.; Negi, T.; Gaur, V.K.; Gnansounou, E.; Bharathiraja, B. Green processing and biotechnological potential of grape pomace: Current trends and opportunities for sustainable biorefinery. Bioresour. Technol. 2020, 314, 123771. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, B.; Yadav, V.; Yadav, A.; Rahman, M.U.; Yuan, W.Z.; Li, Z.; Wang, X. Integrated biorefinery approach to valorize winery waste: A review from waste to energy perspectives. Sci. Total Environ. 2020, 719, 137315. [Google Scholar] [CrossRef]
- Rodrigues, R.P.; Gando-Ferreira, L.M.; Quina, M.J. Increasing Value of Winery Residues through Integrated Biorefinery Processes: A Review. Molecules 2022, 27, 4709. [Google Scholar] [CrossRef] [PubMed]
- Spinei, M.; Oroian, M. The Potential of Grape Pomace Varieties as a Dietary Source of Pectic Substances. Foods 2021, 10, 867. [Google Scholar] [CrossRef]
- Coelho, M.; Pereira, R.; Rodrigues, A.; Teixeira, J.; Pintado, M. The use of emergent technologies to extract added value compounds from grape by-products. Trends Food Sci. Technol. 2020, 106, 182–197. [Google Scholar] [CrossRef]
- Chiurciu, I.-A.; Zaharia, I.; Soare, E. Production of wine grapes and cultural traditions related to vine in Romania. Sci. Pap. Ser. Manag. Econ. Eng. Agric. Rural Dev. 2020, 20, 133–143. [Google Scholar]
- Fuciu, M. New Marketing Tendencies in the Romanian Wine Industry. Stud. Bus. Econ. 2020, 15, 31–39. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef]
- Liazid, A.; Schwarz, M.; Varela, R.M.; Palma, M.; Guillén, D.A.; Brigui, J.; Macías, F.A.; Barroso, C.G. Evaluation of various extraction techniques for obtaining bioactive extracts from pine seeds. Food Bioprod. Process. 2010, 88, 247–252. [Google Scholar] [CrossRef]
- Cavalloro, V.; Martino, E.; Linciano, P.; Collina, S. Microwave-Assisted Solid Extraction from Natural Matrices. In Microwave Heating—Electromagnetic Fields Causing Thermal and Non-Thermal Effects; Churyumov, G.I., Ed.; IntechOpen: Rijeka, Croatia, 2021; Chapter 2. [Google Scholar]
- Li, Y.; Radoiu, M.; Fabiano-Tixier, A.-S.; Chemat, F. From Laboratory to Industry: Scale-Up, Quality, and Safety Consideration for Microwave-Assisted Extraction. In Microwave-Assisted Extraction for Bioactive Compounds: Theory and Practice; Chemat, F., Cravotto, G., Eds.; Springer: Boston, MA, USA, 2013; pp. 207–229. [Google Scholar]
- Savun-Hekimoğlu, B. A Review on Sonochemistry and Its Environmental Applications. Acoustics 2020, 2, 766–775. [Google Scholar] [CrossRef]
- Mason, T.M.; Vinatoru, M. Power ultrasound in food technology. In Sonochemistry; Mason, T.M., Ed.; De Gruyter: Berlin, Germany; Boston, MA, USA, 2023; pp. 161–212. [Google Scholar]
- Hujjatul Islam, M.; Paul, M.T.Y.; Burheim, O.S.; Pollet, B.G. Recent developments in the sonoelectrochemical synthesis of nanomaterials. Ultrason. Sonochem. 2019, 59, 104711. [Google Scholar] [CrossRef] [PubMed]
- Calvete-Torre, I.; Muñoz-Almagro, N.; Pacheco, M.T.; Antón, M.J.; Dapena, E.; Ruiz, L.; Margolles, A.; Villamiel, M.; Moreno, F.J. Apple pomaces derived from mono-varietal Asturian ciders production are potential source of pectins with appealing functional properties. Carbohydr. Polym. 2021, 264, 117980. [Google Scholar] [CrossRef] [PubMed]
- Marić, M.; Grassino, A.N.; Zhu, Z.; Barba, F.J.; Brnčić, M.; Rimac Brnčić, S. An overview of the traditional and innovative approaches for pectin extraction from plant food wastes and by-products: Ultrasound-, microwaves-, and enzyme-assisted extraction. Trends Food Sci. Technol. 2018, 76, 28–37. [Google Scholar] [CrossRef]
- Adetunji, L.R.; Adekunle, A.; Orsat, V.; Raghavan, V. Advances in the pectin production process using novel extraction techniques: A review. Food Hydrocoll. 2017, 62, 239–250. [Google Scholar] [CrossRef]
- Megías-Pérez, R.; Ferreira-Lazarte, A.; Villamiel, M. Valorization of Grape Pomace as a Renewable Source of Techno-Functional and Antioxidant Pectins. Antioxidants 2023, 12, 957. [Google Scholar] [CrossRef]
- Gupta, S.; Doležal, K.; Kulkarni, M.G.; Balázs, E.; Van Staden, J. Role of non-microbial biostimulants in regulation of seed germination and seedling establishment. Plant Growth Regul. 2022, 97, 271–313. [Google Scholar] [CrossRef]
- Ferrari, S.; Savatin, D.V.; Sicilia, F.; Gramegna, G.; Cervone, F.; Lorenzo, G.D. Oligogalacturonides: Plant damage-associated molecular patterns and regulators of growth and development. Front. Plant Sci. 2013, 4, 49. [Google Scholar] [CrossRef]
- Benedetti, M.; Pontiggia, D.; Raggi, S.; Cheng, Z.; Scaloni, F.; Ferrari, S.; Ausubel, F.M.; Cervone, F.; De Lorenzo, G. Plant immunity triggered by engineered in vivo release of oligogalacturonides, damage-associated molecular patterns. Proc. Natl. Acad. Sci. USA 2015, 112, 5533–5538. [Google Scholar] [CrossRef]
- Udchumpisai, W.; Uttapap, D.; Wandee, Y.; Kotatha, D.; Rungsardthong, V. Promoting effect of pectic-oligosaccharides produced from pomelo peel on rice seed germination and early seedling growth. J. Plant Growth Regul. 2023, 42, 2176–2188. [Google Scholar] [CrossRef]

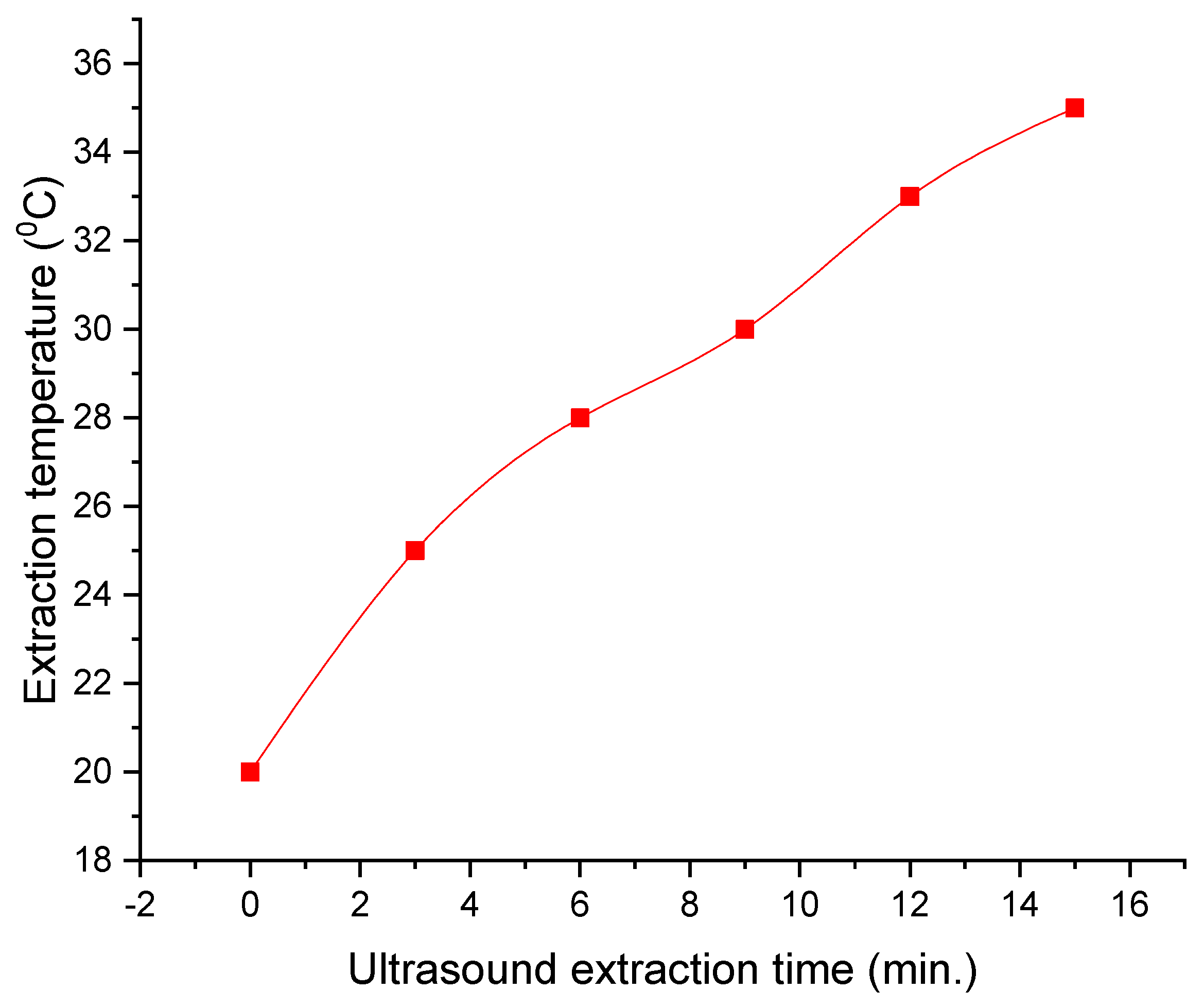

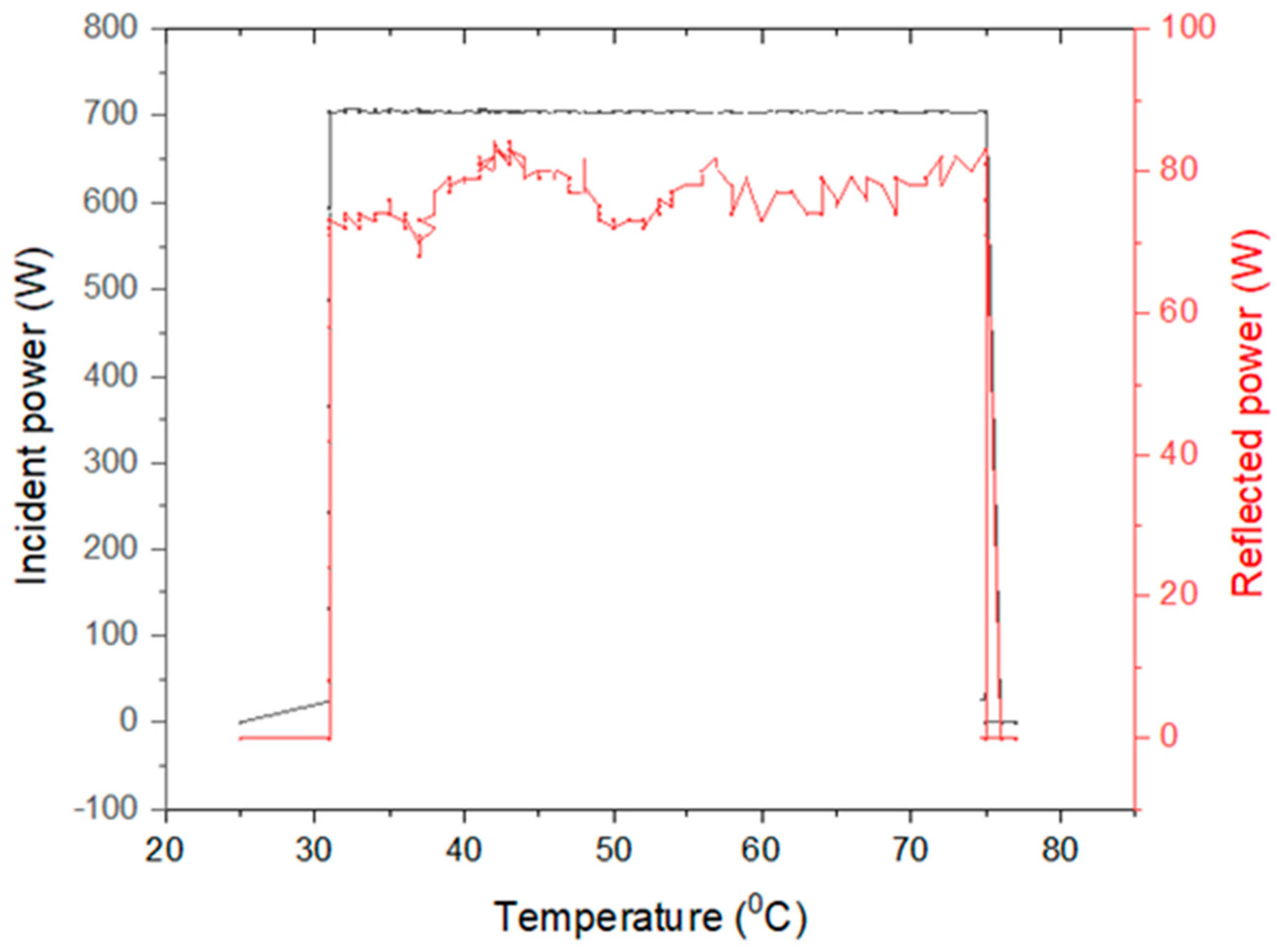
| Ratio H2O/ Citric Acid | Ratio Grape Marc/ Solvent | Ultrasonic Parameters | Microwave- Assisted Extraction Parameters | Centrifugation Parameters | Drying Parameters |
|---|---|---|---|---|---|
| 100:1 | 1:4 | ν = 27 Hz, A = 2.7 A | PMW = 350 W/L, σMW = 30′/L, Extraction = 75 °C | 15 min. 6000× g | 24 h at 50 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patrascu, M.; Kumbakisaka, S.; Oancea, F. The Effect of Microwave and Ultrasound Process Intensification on the Antioxidant Activity of Pectin Extracted from Grape Marc. Chem. Proc. 2023, 13, 11. https://doi.org/10.3390/chemproc2023013011
Patrascu M, Kumbakisaka S, Oancea F. The Effect of Microwave and Ultrasound Process Intensification on the Antioxidant Activity of Pectin Extracted from Grape Marc. Chemistry Proceedings. 2023; 13(1):11. https://doi.org/10.3390/chemproc2023013011
Chicago/Turabian StylePatrascu, Mariana, Sylviu Kumbakisaka, and Florin Oancea. 2023. "The Effect of Microwave and Ultrasound Process Intensification on the Antioxidant Activity of Pectin Extracted from Grape Marc" Chemistry Proceedings 13, no. 1: 11. https://doi.org/10.3390/chemproc2023013011
APA StylePatrascu, M., Kumbakisaka, S., & Oancea, F. (2023). The Effect of Microwave and Ultrasound Process Intensification on the Antioxidant Activity of Pectin Extracted from Grape Marc. Chemistry Proceedings, 13(1), 11. https://doi.org/10.3390/chemproc2023013011







