Antibacterial Activity of Ag2O/SrO/CaO Nanocomposite †
Abstract
1. Introduction
2. Experimental Section
2.1. Peparation of Ag2O/SrO/CaO Nanocomposite
2.2. Characterization
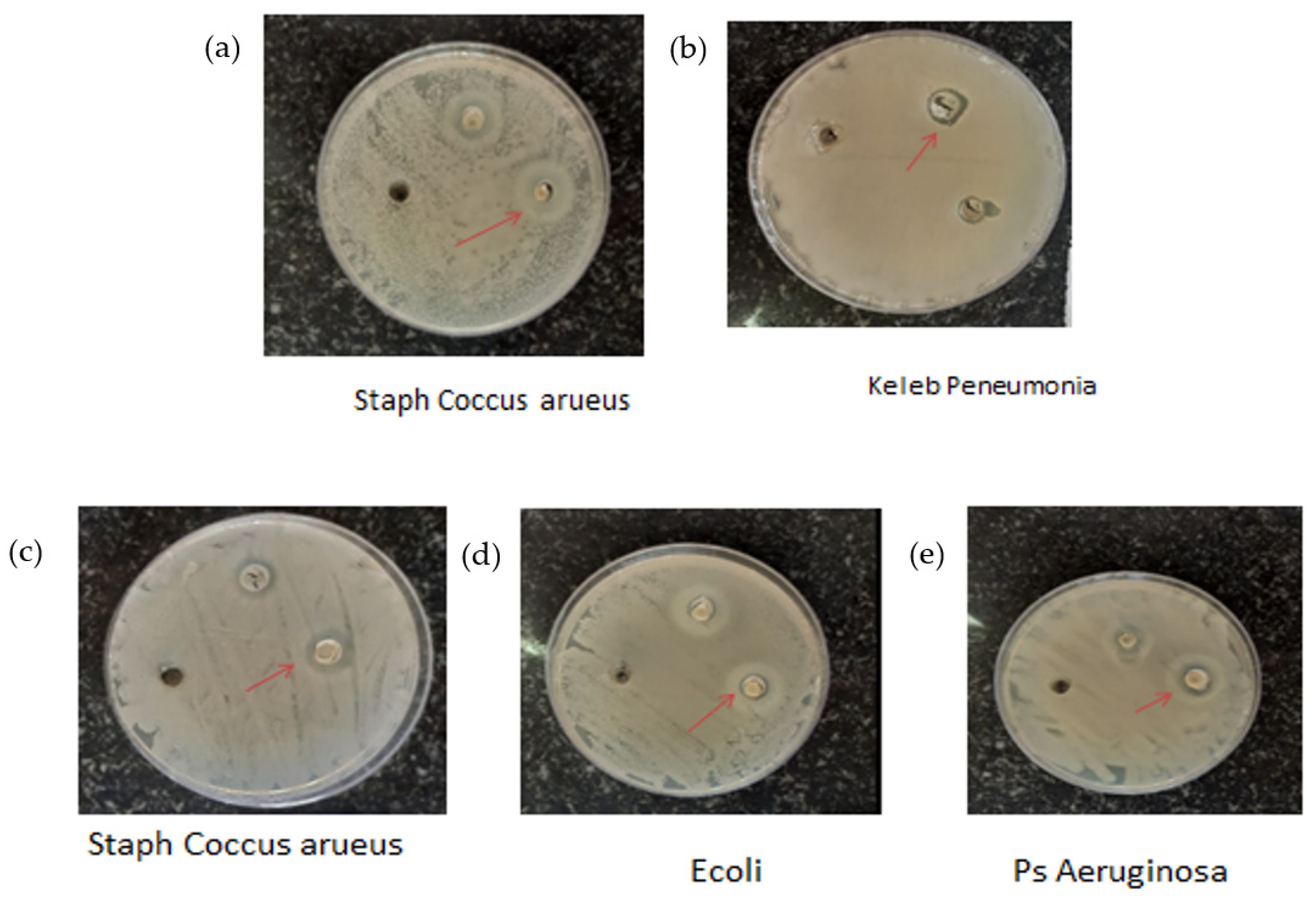
2.3. Antibacterial Activity
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wyszogrodzka, G.; Marszałek, B.; Gil, B.; Dorożyński, P. Metal-organic frameworks: Mechanisms of antibacterial action and potential applications. Drug Discov. Today 2016, 21, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Durán, N.; Durán, M.; De Jesus, M.B.; Seabra, A.B.; Fávaro, W.J.; Nakazato, G. Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Subhan, M.A.; Rifat, T.P.; Saha, P.C.; Alam, M.M.; Asiri, A.M.; Rahman, M.M.; Uddin, J. Enhanced visible light-mediated photocatalysis, antibacterial functions and fabrication of a 3-chlorophenol sensor based on ternary Ag2O·SrO·CaO. RSC Adv. 2020, 10, 11274–11291. [Google Scholar] [CrossRef] [PubMed]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef] [PubMed]
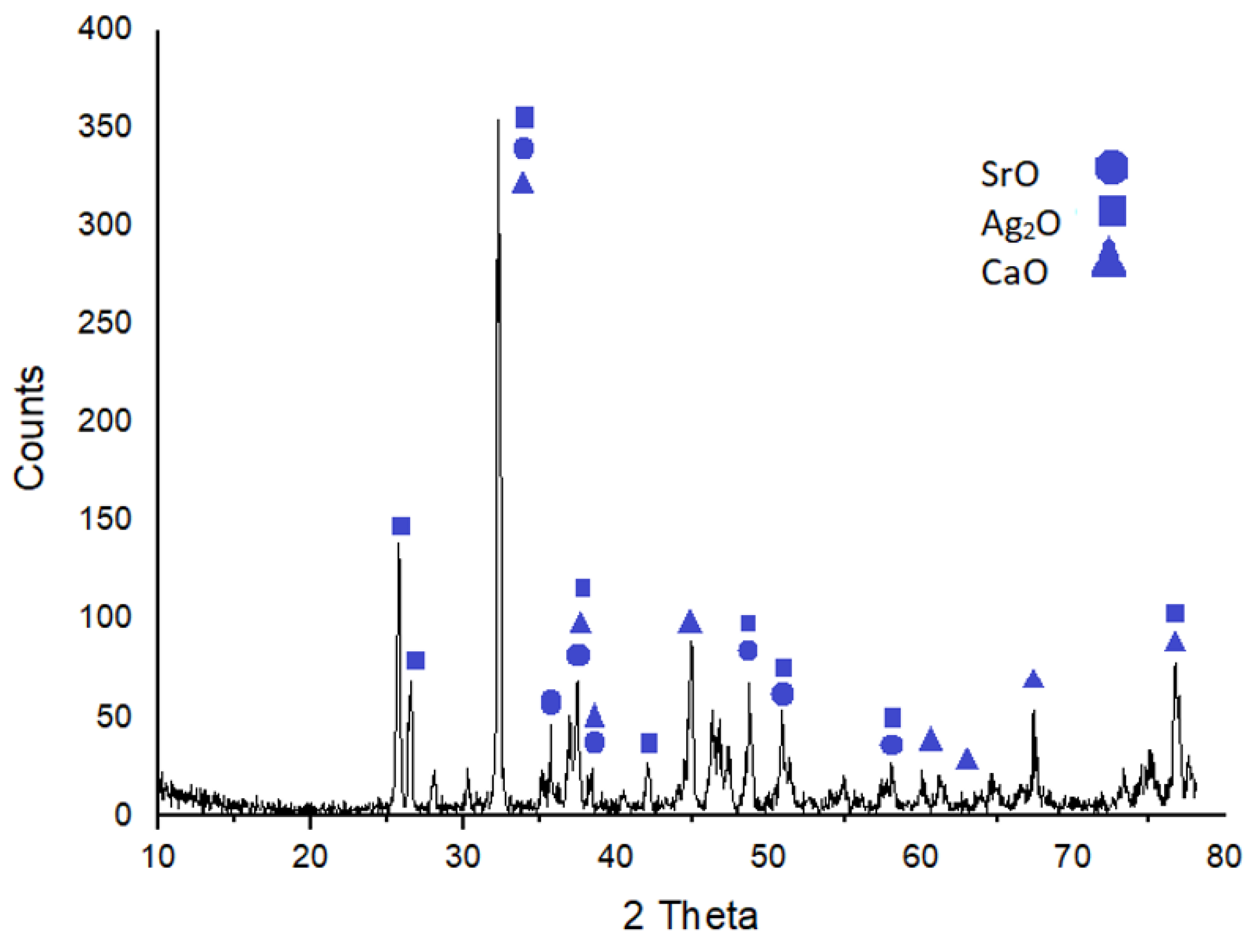

| Elements | Ag | Na | Al2O3 | SiO2 | P2O5 | SO2 | K2O | CaO | TiO2 |
| wt% | 56.062 | >> | – | – | – | – | – | 9.627 | – |
| Elements | Fe2O3 | V2O5 | MnO | Cr2O3 | Ba | Sr | Zn | Se | Nb |
| wt% | – | – | – | – | – | 31.985 | – | – | >> |
| Elements | F | Cr | Cl | Ce | Co | Mo | Ca | Cu | Ho |
| wt% | – | – | 2.325 | – | – | – | – | >> | – |
| Test Bacteria | Inhibition Zone Diameter (mm) |
|---|---|
| Ag2O/SrO/CaO Nano Composite | |
| Ps. Aeruginosa | 18.991 |
| Keleb Peneumonia | 7.876 |
| Staph Coccus aureus | 13.785 |
| Staph Sapropphyticus | 12.723 |
| E. coli | 16.456 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aghaee, M.; Manteghi, F. Antibacterial Activity of Ag2O/SrO/CaO Nanocomposite. Chem. Proc. 2022, 12, 77. https://doi.org/10.3390/ecsoc-26-13577
Aghaee M, Manteghi F. Antibacterial Activity of Ag2O/SrO/CaO Nanocomposite. Chemistry Proceedings. 2022; 12(1):77. https://doi.org/10.3390/ecsoc-26-13577
Chicago/Turabian StyleAghaee, Mina, and Faranak Manteghi. 2022. "Antibacterial Activity of Ag2O/SrO/CaO Nanocomposite" Chemistry Proceedings 12, no. 1: 77. https://doi.org/10.3390/ecsoc-26-13577
APA StyleAghaee, M., & Manteghi, F. (2022). Antibacterial Activity of Ag2O/SrO/CaO Nanocomposite. Chemistry Proceedings, 12(1), 77. https://doi.org/10.3390/ecsoc-26-13577





