Reactions of exo-exo-hexacyclo[9.2.1.02,10.03,8.04,6.05,9]tetradecane with Aliphatic Alcohols under the Action of Ionic Liquids †
Abstract
1. Introduction
2. Experimental Section
2.1. General Procedures and Materials
2.2. Preparation of Ionic Liquids
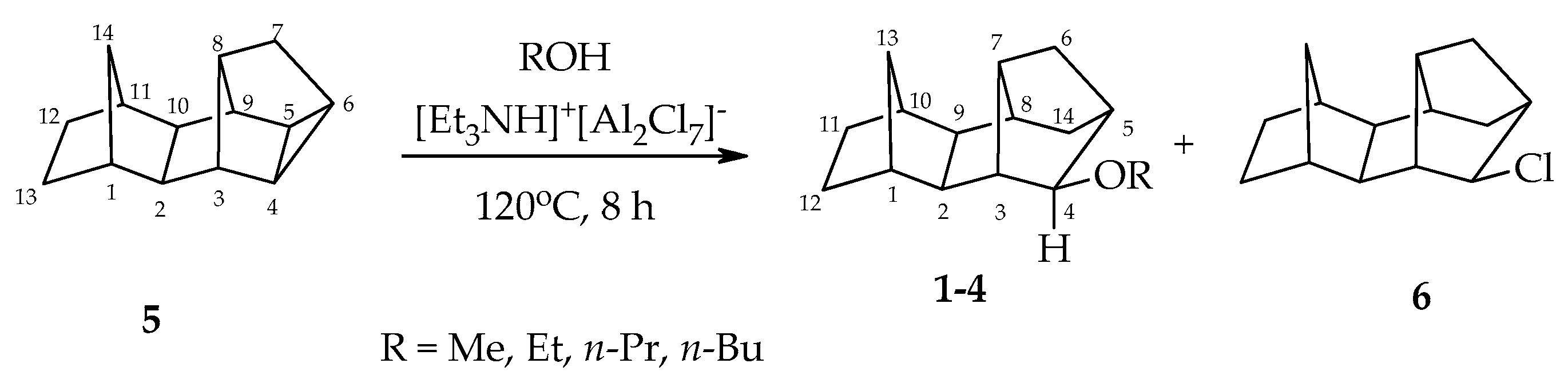
2.3. General Procedure for the Preparation of 4-exo-alkoxypentacyclo[8.2.1.15,8.02,9.03,7]tetradecanes
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qiao, K.; Deng, Y. Alkylations of benzene in room temperature ionic liquids modified with HCl. J. Mol. Catal. A Chem. 2001, 17, 81–84. [Google Scholar] [CrossRef]
- Xin, H.; Wu, Q.; Han, M.; Wang, D.; Jin, Y. Alkylation of benzene with 1-dodecene in ionic liquids [Rmim]+Al2Cl6X− (R = butyl, octyl and dodecyl; X = chlorine, bromine and iodine). Appl. Catal. A 2005, 292, 354–361. [Google Scholar] [CrossRef]
- Sun, X.W.; Thao, S.Q. Revealing the catalytic mechanism of an ionic liquid with an isotope exchange method. Pet. Sci. 2011, 8, 495–501. [Google Scholar] [CrossRef][Green Version]
- Kim, D.S.; Ahn, W.S. Diphenylmethane synthesis using ionic liquids as lewis acid catalyst. Korean J. Chem. Eng. 2003, 20, 39–43. [Google Scholar] [CrossRef]
- Xin-hua, Y.; Min, C.; Qi-xun, D.; Xiao-nong, C. Friedel–Crafts acylation of anthracene with oxalyl chloride catalyzed by ionic liquid of [bmim]Cl/AlCl3. Chem. Eng. J. 2009, 146, 266–269. [Google Scholar] [CrossRef]
- Yoo, K.; Namboodiri, V.V.; Varma, R.S.; Smimiotis, P.G. Ionic liquid-catalyzed alkylation of isobutane with 2-butene. J. Catal. 2004, 222, 511–519. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, C.; Chen, B.; Ren, P.; Pu, M. Isobutane/2-butene alkylation catalyzed by chloroaluminate ionic liquids in the presence of aromatic additives. J. Catal. 2007, 249, 261–268. [Google Scholar] [CrossRef]
- Rongan, X.; Rui, Z.; Xianghai, M.; Zhichang, L.; Jiaying, M.; Chunming, X. Chloroaluminate ionic liquid catalyzed isomerization of n-pentane in the presence of product distribution improver. Pet. Sci. 2011, 8, 219–223. [Google Scholar] [CrossRef][Green Version]
- Huang, M.Y.; Wu, J.C.; Shieu, F.S.; Lin, J.J. Preparation of high energy fuel JP-10 by acidity-adjustable chloroaluminate ionic liquid catalyst. Fuel 2011, 90, 1012–1017. [Google Scholar] [CrossRef]
- Huang, M.Y.; Wu, J.C.; Shieu, F.S.; Lin, J.J. Isomerization of exo-tetrahydrodicyclopentadiene to adamantane using an acidity-adjustable chloroaluminate ionic liquid. Catal. Commun. 2009, 10, 1747–1751. [Google Scholar] [CrossRef]
- Khusnutdinov, R.I.; Mukminov, R.R.; Aminov, R.I.; Khalilov, L.M.; Mescheryakova, E.S.; Dzhemilev, U.M. Synthesis and X-ray diffraction study of triamantane. Tetrahedron Lett. 2015, 56, 536–538. [Google Scholar] [CrossRef]
- Aminov, R.I.; Khusnutdinov, R.I. Synthesis of diamantane via skeletal isomerization of hydrogenated cyclohepta-1,3,5-triene dimers in ionic liquid [Et3NH]+[Al2Cl7]–. Russ. J. Org. Chem. 2017, 53, 1881–1883. [Google Scholar] [CrossRef]
- Khusnutdinov, R.I.; Aminov, R.I.; Egorova, T.M.; Khalilov, L.M.; Mescheryakova, E.S.; Dzhemilev, U.M. Alkylation of Benzene with Cyclopropane-Containing Polycyclic Hydrocarbons under the Action of the [Et3NH]+[Al2Cl7]– Ionic Liquid. Chem. Select. 2018, 3, 9600–9602. [Google Scholar] [CrossRef]
- Aminov, R.I.; Mazitova, A.S.; Khusnutdinov, R.I. Benzene Alkylation with Cycloolefins under the Action of [Et3NH]+[Al2Cl7]− Ionic Liquid. Russ. J. Gen. Chem. 2019, 89, 2171–2177. [Google Scholar] [CrossRef]
- Aminov, R.I.; Akshieva, A.N.; Khusnutdinov, R.I. Hydroisomerization of hexacyclo[9.2.1.02,10.03,8.04,6.05,9]tetradecanes to diamantane induced by ionic liquids. Catal. Commun. 2019, 130, 105756. [Google Scholar] [CrossRef]
- Aminov, R.I.; Khusnutdinov, R.I. Synthesis of polycyclic hydrocarbons C14H20 by hydrogenation of exo-exo-, exo-endo-, endo-exo-, and endo-endo- hexacyclo[9.2.1.02,10.03,8.04,6.05,9]tetradec-12-enes with H2SO4 and isomerization of the products to diamantane induced by ionic liquids. Ind. Eng. Chem. Res. 2021, 60, 12776–12782. [Google Scholar] [CrossRef]
- Myers, H.K.; Schneider, A.; Suld, G. Dimerization of Norbornadiene to an Exo-exo Hexacyclic Dimer. USA Patent US4207080, 10 June 1980. [Google Scholar]
- Aminov, R.I.; Khusnutdinov, R.I. Alcoholysis of Binor-S with Alcohols under the Action of Ionic Liquid. Russ. J. Org. Chem. 2019, 55, 587–591. [Google Scholar] [CrossRef]
- Aminov, R.I.; Karimova, I.M.; Khusnutdinov, R.I. Reactions of Binor-S with α,ω-Diols and Organic Acids in the Presence of Inorganic Ionic Liquids. Russ. J. Org. Chem. 2020, 56, 1595–1599. [Google Scholar] [CrossRef]
| ROH | Ratio 5:IL | Yield, % | |
|---|---|---|---|
| 1–4 | 6 | ||
| MeOH | 1:1 | 33 | 44 |
| EtOH | 1:1 | 36 | 42 |
| n-PrOH | 1:1 | 32 | 39 |
| n-BuOH | 1:1 | 29 | 38 |
| MeOH | 5:1 | 49 | 31 |
| EtOH | 5:1 | 51 | 27 |
| n-PrOH | 5:1 | 50 | 26 |
| n-BuOH | 5:1 | 49 | 23 |
| MeOH | 10:1 | 88 | 12 |
| EtOH | 10:1 | 89 | 11 |
| n-PrOH | 10:1 | 88 | 12 |
| n-BuOH | 10:1 | 87 | 13 |
| MeOH | 30:1 | 36 | 10 |
| EtOH | 30:1 | 39 | 12 |
| n-PrOH | 30:1 | 33 | 9 |
| n-BuOH | 30:1 | 31 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aminov, R.I.; Ramazanov, I.R. Reactions of exo-exo-hexacyclo[9.2.1.02,10.03,8.04,6.05,9]tetradecane with Aliphatic Alcohols under the Action of Ionic Liquids. Chem. Proc. 2022, 12, 6. https://doi.org/10.3390/ecsoc-26-13584
Aminov RI, Ramazanov IR. Reactions of exo-exo-hexacyclo[9.2.1.02,10.03,8.04,6.05,9]tetradecane with Aliphatic Alcohols under the Action of Ionic Liquids. Chemistry Proceedings. 2022; 12(1):6. https://doi.org/10.3390/ecsoc-26-13584
Chicago/Turabian StyleAminov, Rishat I., and Ilfir R. Ramazanov. 2022. "Reactions of exo-exo-hexacyclo[9.2.1.02,10.03,8.04,6.05,9]tetradecane with Aliphatic Alcohols under the Action of Ionic Liquids" Chemistry Proceedings 12, no. 1: 6. https://doi.org/10.3390/ecsoc-26-13584
APA StyleAminov, R. I., & Ramazanov, I. R. (2022). Reactions of exo-exo-hexacyclo[9.2.1.02,10.03,8.04,6.05,9]tetradecane with Aliphatic Alcohols under the Action of Ionic Liquids. Chemistry Proceedings, 12(1), 6. https://doi.org/10.3390/ecsoc-26-13584






