Abstract
Currently, world population faces an episode where bacteria are becoming resistant to antibiotics, and it is crucial to find alternatives and new molecules to fight these microorganisms. Photodynamic inactivation of microorganisms has been pointed out as an alternative to conventional therapies. This work describes the synthesis of benzoporphyrins derivatives bearing triazolyl groups and of the analogues with pyridyl units under Heck coupling conditions. The benzoporphyrin derivatives containing pyridyl groups were further quaternized with iodomethane and 1-iodopentane to evaluate the influence of alkyl chain size on their photoinactivation ability. The biological studies towards Gram-negative Escherichia coli showed that the tetracationic benzoporphyrins can efficiently inactivate this bacterium.
1. Introduction
Porphyrins are tetrapyrrolic macrocycles with important roles in some biological processes such as respiration or electron transportation. Due to their unique physicochemical properties, porphyrins are currently being used in a wide range of applications including artificial photosynthesis, catalysis, sensors, nonlinear optics, and medicine [1,2]. In this specific field, considerable attention is being given to their use as photosensitizers (PS) in photodynamic therapy (PDT) of cancer [3,4] or, more recently, in the photoinactivation of microorganisms usually referred to as antimicrobial photodynamic therapy (aPDT) [5,6].
Similar to PDT, aPDT requires the presence of a PS, light, and dioxygen (3O2). The association of these components results in the formation of reactive oxygen species (ROS), such as singlet oxygen (1O2) that cause damage to vital biomolecules leading to microbial death [7,8,9]. The multitarget action of aPDT supports its selection as an excellent alternative to conventional antimicrobial drugs considering the emergence of microbial resistance to many of those therapeutic agents used in clinical and environmental contexts [10]. It has been shown that the type of bacterial membrane and the PS ability to bind to it are factors that influence antimicrobial photoinactivation [8].
Due to the chemical and photophysical properties of porphyrins, this family of compounds is considered the foremost used as PSs in aPDT [11]. Therefore, it is crucial to develop novel synthetic approaches or to improve the outcomes of the ones already described to provide porphyrin derivatives with suitable characteristics for their application in aPDT. In this work, we report the preparation under conventional and microwave (MW) heating of benzoporphyrins derivatives bearing triazolyl groups and of the analogues with pyridyl units under Heck coupling conditions. The neutral pyridyl derivatives were then submitted to cationization by using appropriate alkylating agents.
2. Experimental
2.1. Synthesis of the Starting Porphyrins and Benzoporphyrin 2a
Starting porphyrins 1a,b and benzoporphyrin derivative 2a were synthesized following well-established procedures already reported in the literature. The structure of these compounds was confirmed by 1H NMR spectroscopy and mass spectrometry and agrees with the analytical data described in the literature [12,13,14,15].
2.2. Synthesis of Benzoporphyrin 2b
2.2.1. Under Conventional Heating Conditions
In a 25 mL flask, the nickel(II) complex 1b (20.3 mg, 0.020 mmol), Pd(OAc)2 (2.3 mg, 0.010 mmol), PPh3 (9.9 mg, 0.038 mmol), K2CO3 (12.2 mg, 0.089 mmol), and 1-phenyl-4-vinyl-1,2,3-triazole (28.0 mg, 0.164 mmol) were added. The solids, under a nitrogen atmosphere, were dissolved in toluene (1 mL) and DMF (0.5 mL). The reaction was kept at 120 °C for 6 h when the thin-layer chromatography (TLC) control showed the total consumption of the starting Ni(II) porphyrin derivative 1b. The mixture was washed with water, and the organic phase was extracted with toluene. The crude was purified by preparative TLC using CHCl3 and MeOH (4:1) as eluent. Triazolo-benzoporphyrin 2b was obtained in 15% yield (3.1 mg).
2.2.2. Under Microwave Irradiation
The same amounts of nickel(II) complex 1b, triazole, and catalytic system used in the conventional heating method were added to a microwave reactor and dissolved in a 2:1 toluene/DMF mixture (1.5 mL). The reaction mixture was subjected to microwave radiation for 5 min at 120 °C, at a power of 250 W in a CEM Microwave Synthesis System Discover. At the end of this time, it was found that all the starting material 1b had been consumed. The reaction was washed with water and the organic phase was extracted with toluene. The reaction mixture was purified by preparative thin layer chromatography using first CHCl3 as eluent and then a mixture of CHCl3/MeOH (4:1). The desired benzoporphyrin 2b bearing triazolyl units was obtained in 10% yield (2.0 mg).
Yield: 10%. 1H NMR (300 MHz, CDCl3): δ 8.73 (s, 4H, β-H), 8.03-8.00 (m, 12H, H-o-Ph and H-5′-Triazole), 7.80-7.77 (m, 20H, H-o-Ph-Triazole and H-m,p-Ph); 7.59 (t, J = 7.6 Hz, 8H, H-m-Ph-Triazole), 7.49 (d, J = 7.6 Hz, 4H, H-p-Ph-Triazole), 7.44 (s, 4H, H-1´). MS-ESI(+): m/z calculated for C84H52N16Ni [M]+• 1344.2; found 1344.0.
2.3. Synthesis of the Cationic Benzoporphyrins 4a,b
The charged benzoporphyrins 4a,b were prepared from the free-base derivative 3 using methyl iodide or 1-iodopentane as alkylating agents following well-established procedures already reported in the literature [16]. The structure of these compounds was confirmed by 1H NMR spectroscopy and mass spectrometry and is in agreement with those described in the literature [13].
3. Results and Discussion
3.1. Synthesis
The synthetic route to access the tetracationic triazolyl and pyridyl porphyrins required the preparation of the 5,10,15,20-tetraphenylporphyrin (TPP) for further bromination at beta pyrrolic positions to attain compound 1a (Scheme 1). These starting porphyrins were prepared following a well-established procedure reported in the literature [13].
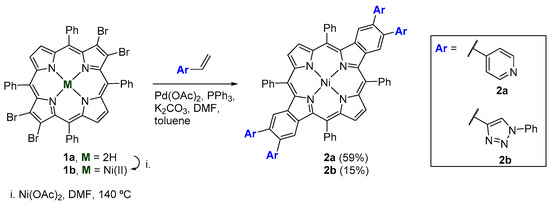
Scheme 1.
Heck reaction conditions used to synthesize benzoporphyrin derivatives 2a,b.
Once the Heck reaction is catalyzed by palladium it was necessary to protect the core of the macrocycle in order to minimize the loss of the catalyst during the catalyst cycle through the coordination of the porphyrin with Pd(II) [17]. Thus, porphyrin 1a was complexed with Ni(II) using nickel(II) acetate in DMF under reflux for 1 h. After the reaction was completed, Ni(II) porphyrinate 1b was obtained with a yield of 67%. The simplification of the UV-Vis spectrum of 1b in the Q band region, when compared with the one of porphyrin 1a (two bands versus four bands) confirmed the success of this complexation step. The first assays concerning the Heck coupling reaction between the nickel(II) porphyrinate 1b and 4-vinylpyridine in the presence of Pd(OAc)2, PPh3, and K2CO3 were performed in a mixture of dimethylformamide (DMF) and toluene under conditions such as those described by Jiang et al. [15]. After 6 h at 120 °C, the TLC monitorization confirmed the full consumption of the starting material (compound 1b) and the formation of several more polar compounds. After work-up and preparative chromatography, the most polar and main compound was identified as the desired derivative 2a and was isolated in 15% yield. In order to minimize the formation of secondary products and to improve the yield of compound 2a, the Heck coupling reaction was repeated but now under microwave irradiation. This experiment was performed in an MW reactor, for 5 min at 120 °C and at a power of 250 W and using the same catalytic conditions previously reported to couple nickel(II) porphyrinate 1b and 4-vinylpyridine. Benzoporphyrin 2a was obtained with a yield of 59%, substantially higher than that obtained in the reaction performed under conventional heating conditions.
Due to the success achieved in the synthesis of benzoporphyrin derivative 2a, this methodology was extended to the reaction between Ni(II) porphyrinate 1b and 1-phenyl-4-vinyl-1,2,3-triazole. Triazoles play an important role in medicinal and organic chemistry due to their fairly simple synthesis, versatility, and remarkable biological activity [18,19]. Firstly, we decided to try the conventional heating approach used by Jiang et al. [15] procedure reported for benzoporphyrin 2a. In this case, the most polar and main product was identified as the desired benzoporphyrin bearing the four triazolyl units and was isolated in 15% yield; unfortunately, many other compounds were obtained from the reaction in amounts that did not allow their identification.
With the main purpose to increase the yield of the reaction and due to the previous success, we decided to perform an optimization study under MW irradiation conditions. In this study, were evaluated several parameters such as time and addition of an oxidizing agent (nitrobenzene) at different reaction temperatures. The different conditions are summarized in Table 1 and the results obtained allowed us to conclude that the better conditions to obtain 2b are under conventional heating conditions followed by MW irradiation for 10 min at 120 °C and in the absence of the oxidant.

Table 1.
Conditions studied aiming to prepare benzoporphyrin derivatives 2b.
Due to the very low yield obtained for triazolyl benzoporphyrin derivative 2b, we decided not to proceed with this compound in the following synthetic steps, as well as with the biological evaluation.
Knowing that the Ni(II) complexes of porphyrins present poor efficiency to generate singlet oxygen, but the presence of positive charges improves the photodynamic inactivation [20], the benzoporphyrin 2a was first demetalled for further cationization.
In order to evaluate the influence of alkyl chain size on singlet oxygen generation and on the photoinactivation of antibiotic-resistant bacteria, the cationization of the pyridyl groups of the benzoporphyrin derivative 3 with iodomethane and 1-iodopentane was performed (Scheme 2); the desired cationic porphyrins 4a and 4b were obtained quantitatively.
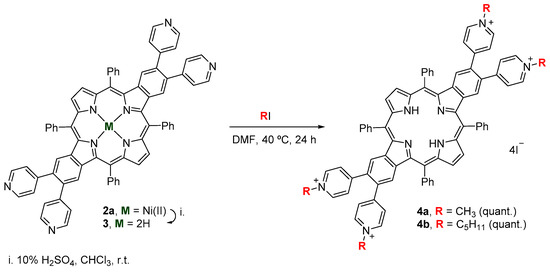
Scheme 2.
Synthetic approach to attain cationic benzoporphyrin derivatives 4a,b.
3.2. Photophysical and Photochemical Properties and Photoinactivation of Bioluminescent E. coli
The UV-Vis absorption spectrum was recorded for each benzoporphyrin. It was possible to conclude that the modifications in the benzoporphyrin core structure influence the absorption spectrum of the different compounds. The electronic spectrum of Ni(II) complex 2a shows the typical sharp band at 444 nm (Soret band) and two less intense bands but well-defined bands at 559 and 598 nm, respectively. The corresponding free-base derivative 3 displays the Soret band at 436 nm and four Q bands ranging from 526 to 670 nm. After quaternization of the pyridyl units, the Soret band of each compound 4a and 4b are red-shifted ca. 30 nm and is possible to observe three Q-bands from 536 to 613 nm.
The evaluation of the stability of a molecule under irradiation is mandatory before evaluating its efficiency to generate singlet oxygen generation. Therefore, benzoporphyrin 3 and tetracationic benzoporphyrins derivatives 4a and 4b were exposed to white light irradiation for 30 min with a power of 11 mW/cm2. The absence of any significative change in their UV-Vis spectra confirmed that these compounds are photostable.
One of the most relevant features of a PS is its aptitude to generate singlet oxygen which is strongly related to the efficiency of the photodynamic process [20]. The capability of the PSs synthesized to generate 1O2 was quantitatively assessed by monitoring the decomposition of 1,3-diphenylisobenzofuran (DPiBF) at 415 nm in DMF/H2O (9:1). Therefore, the absorbance decrease in DPiFB allowed us to evaluate the generation of 1O2 by the PS present in solution [21].
The data obtained with neutral benzoporphyrin derivative 3 and tetracationic benzoporphyrins derivatives 4a and 4b (Figure 1) confirmed their ability to induce the decay of DPiFB absorption when irradiated with red light in the presence of each PS. In addition, tetracationic benzoporphyrin derivative 4b showed a superior 1O2 production capacity than Tetra-Py(+)-Me, a reference compound pointed out as a good 1O2 generator [22].
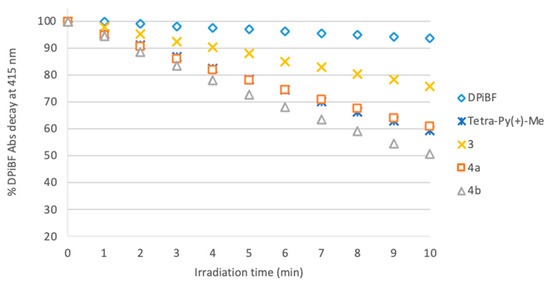
Figure 1.
Time-dependent photodecomposition of DPiBF at 50 µM photosensitized by derivatives 3, 4a,b and Tetra-Py(+)-Me at 0.5 µM in DMF/H2O (9:1) upon irradiation with red light (630 ± 20 nm) at an irradiation of 11 mW/cm2.
Preliminary studies show that both tetracationic benzoporphyrin derivatives 4a and 4b display the capability to act as a PS in the photoinactivation of bioluminescent gram-negative E. coli. The derivative with pentyl chain showed to be more efficient in E. coli photoinactivation when compared to the methyl porphyrin, reaching the detection limit of the method after 180 min of irradiation, probably due to its longer alkyl chain, which promotes better bacterial membrane binding.
4. Conclusions
This work describes a synthetic approach that allowed for the first time to prepare a benzoporphyrin derivative bearing triazolyl moieties via Heck reaction coupling reaction. However, the low yield obtained makes mandatory further synthetic studies to improve the yield of this derivative. The yield of the benzoporphyrin derivative bearing pyridyl units at beta-pyrrolic positions was improved under microwave irradiation and the products obtained after quaternization of the pyridyl units with appropriate alkylating agents, display the requirements and potential to be used as photosensitizers in the photoinactivation of microorganisms.
Author Contributions
Conceptualization, N.M.M.M. and A.T.P.C.G.; methodology, N.M.M.M. and A.T.P.C.G.; validation, M.A.F.F., A.A. and M.G.P.M.S.N.; formal analysis, N.M.M.M. and A.T.P.C.G.; investigation, F.M.P.M. and C.V.; resources, M.A.F.F. and A.A.; writing—original draft preparation, F.M.P.M. and N.M.M.M.; writing—review and editing, M.A.F.F., A.A. and M.G.P.M.S.N.; supervision, A.A. and M.G.P.M.S.N. All authors have read and agreed to the published version of the manuscript.
Funding
This work received financial support from PT national funds (FCT/MCTES, Fundação para a Ciência e Tecnologia and Ministério da Ciência, Tecnologia e Ensino Superior) through the projects LAQV-REQUIMTE (UIDB/50006/2020 and UIDP/50006/2020) and CESAM (UIDP/50017/2020+UIDB/50017/2020), and the FCT project POR2PS (FCT-EXPL/QUI-QOR/0586/2021). C Vieira thanks FCT for their doctoral grant (SFRH/PD/150358/2019). NMM Moura thanks FCT (Fundação para a Ciência e Tecnologia) for funding through program DL 57/2016—Norma transitória (Ref. CDL-CTTRI-048-88-ARH/2018).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the findings of this study are available within the paper and from the corresponding authors upon request.
Acknowledgments
The work was supported by University of Aveiro and FCT/MCTES through the projects UIDB/50006/2020 and UIDP/50006/2020 (LAQV-REQUIMTE) and (UIDP/50017/2020 and UIDB/50017/2020 (CESAM), funded by FCTFCT/MCTES through national funds, and to the Portuguese NMR Network.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kadish, K.M.; Smith, K.M.; Guilard, R. Handbook of Porphyrin Science; World Scientific Publishing Company: Singapore, 2010. [Google Scholar]
- Vallejo, M.C.S.; Moura, N.M.M.; Gomes, A.T.P.C.; Joaquinito, A.S.M.; Faustino, M.A.F.; Almeida, A.; Gonçalves, I.; Serra, V.V.; Neves, M.G.P.M.S. The role of porphyrinoid photosensitizers for skin wound healing. Int. J. Mol. Sci. 2021, 22, 4121. [Google Scholar] [CrossRef] [PubMed]
- Dias, C.J.; Sardo, I.; Moura, N.M.M.; Felgueiras, J.; Neves, M.G.P.M.S.; Fardilha, M.; Faustino, A.F. An efficient synthetic access to new uracil-alditols bearing a porphyrin unit and biological assessment in prostate cancer cells. Dye. Pigment. 2020, 173, 107996. [Google Scholar] [CrossRef]
- Gamelas, S.R.D.; Moura, N.M.M.; Habraken, Y.; Piette, J.; Neves, M.G.P.M.S.; Faustino, M.A.F. Tetracationic porphyrin derivatives against human breast cancer. J. Photochem. Photobiol. B Biol. 2021, 222, 112258. [Google Scholar] [CrossRef] [PubMed]
- Eddahmi, M.; Moura, N.M.M.; Bouissane, L.; Amiri, O.; Faustino, M.A.F.; Cavaleiro, J.A.S.; Mendes, R.F.; Paz, F.A.A.; Neves, M.G.P.M.S.; Rakib, E.M. A suitable functionalization of nitroindazoles with triazolyl and pyrazolyl moieties via cycloaddition reactions. Molecules 2020, 25, 126. [Google Scholar] [CrossRef]
- Wen, X.; Li, Y.; Hamblin, M.R. Photodynamic therapy in dermatology beyond non-melanoma cancer: An update. Photodiagnosis Photodyn. Ther. 2017, 19, 140–152. [Google Scholar] [CrossRef]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef]
- do Prado-Silva, L.; Brancini, G.T.P.; Braga, G.Ú.L.; Liao, X.; Ding, T.; Sant’Ana, A.S. Antimicrobial photodynamic treatment (aPDT) as an innovative technology to control spoilage and pathogenic microorganisms in agri-food products: An updated review. Food Control 2022, 132, 108527. [Google Scholar] [CrossRef]
- Yang, B.; Chen, Y.; Shi, J. Reactive Oxygen Species (ROS)-Based Nanomedicine. Chem. Rev. 2019, 119, 4881–4985. [Google Scholar] [CrossRef]
- Hou, J.; Long, X.; Wang, X.; Li, L.; Mao, D.; Luo, Y.; Ren, H. Global trend of antimicrobial resistance in common bacterial pathogens in response to antibiotic consumption. J. Hazard. Mater. 2023, 442, 130042. [Google Scholar] [CrossRef]
- Wang, X.; Lv, H.; Sun, Y.; Zu, G.; Zhang, X.; Song, Y.; Zhao, F.; Wang, J. New porphyrin photosensitizers—Synthesis, singlet oxygen yield, photophysical properties and application in PDT. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 279, 121447. [Google Scholar] [CrossRef]
- Kumar, P.K.; Bhyrappa, P.; Varghese, B. An improved protocol for the synthesis of antipodal β-tetrabromo-tetraphenylporphyrin and the crystal structure of its Zn(II) complex. Tetrahedron Lett. 2003, 44, 4849–4851. [Google Scholar] [CrossRef]
- Menilli, L.; Monteiro, A.R.; Lazzarotto, S.; Morais, F.M.P.; Gomes, A.T.P.C.; Moura, N.M.M.; Fateixa, S.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Trindade, T.; et al. Graphene oxide and graphene quantum dots as delivery systems of cationic porphyrins: Photo-antiproliferative activity evaluation towards t24 human bladder cancer cells. Pharmaceutics 2021, 13, 1512. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zaenglein, R.A.; Engle, J.T.; Mittal, C.; Hartley, C.S.; Ziegler, C.J.; Wang, H. Water-soluble ionic benzoporphyrins. Chem. Commun. 2012, 48, 6927–6929. [Google Scholar] [CrossRef]
- Deshpande, R.; Jiang, L.; Schmidt, G.; Rakovan, J.; Wang, X.; Wheeler, K.; Wang, H. A Concise Approach to the Synthesis of opp-Dibenzoporphyrins through the Heck Reaction. Org. Lett. 2009, 11, 4251–4253. [Google Scholar] [CrossRef]
- Vallejo, M.C.S.; Reis, M.J.A.; Pereira, A.M.V.M.; Serra, V.V.; Cavaleiro, J.A.S.; Moura, N.M.M.; Neves, M.G.P.M.S. Merging pyridine(s) with porphyrins and analogues: An overview of synthetic approaches. Dye. Pigment. 2021, 191, 109298. [Google Scholar] [CrossRef]
- Alisha, M.; Philip, R.M.; Anilkumar, G. An overview of rhodium-catalysed heck type reactions. J. Organomet. Chem. 2022, 959, 122207. [Google Scholar] [CrossRef]
- Moura, N.M.M.; Tomé, A.C. 1,2,3-Triazoles. Compr. Heterocycl. Chem. IV 2022, 5, 1–77. [Google Scholar] [CrossRef]
- Abdelli, A.; Azzouni, S.; Plais, R.; Gaucher, A.; Efrit, M.L.; Prim, D. Recent advances in the chemistry of 1,2,4-triazoles: Synthesis, reactivity and biological activities. Tetrahedron Lett. 2021, 86, 153518. [Google Scholar] [CrossRef]
- Moreira, X.; Santos, P.; Faustino, M.A.F.; Raposo, M.M.M.; Costa, S.P.G.; Moura, N.M.M.; Gomes, A.T.P.C.; Almeida, A.; Neves, M.G.P.M.S. An insight into the synthesis of cationic porphyrin-imidazole derivatives and their photodynamic inactivation efficiency against Escherichia coli. Dye. Pigment. 2020, 178, 108330. [Google Scholar] [CrossRef]
- Greci, L.; Kaczor, J.; Carloni, P.; Damiani, E.; Greci, L.; Stipa, P.; Tanfani, F.; Tartaglini, E.; Wozniak, M. On the use of 1,3-diphenylisobenzofuran (DPBF). reactions with carbon and oxygen centered radicals in model and natu-ral systems SYSTEMS. Res. Clwm. Lntermed 1993, 19, 395–405. [Google Scholar]
- Alves, E.; Costa, L.; Carvalho, C.M.; Tomé, J.P.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.; Cunha, Â.; Almeida, A. Charge Effect on the Photoinactivation of Gram-Negative and Gram-Positive Bacteria by Cationic Meso-Substituted Porphyrins. BMC Microbiol. 2009, 9, 70. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).