Organophosphorus Chemistry: Synthesis of New Phosphonic Acid Derivatives Bearing a Triazole Moiety †
Abstract
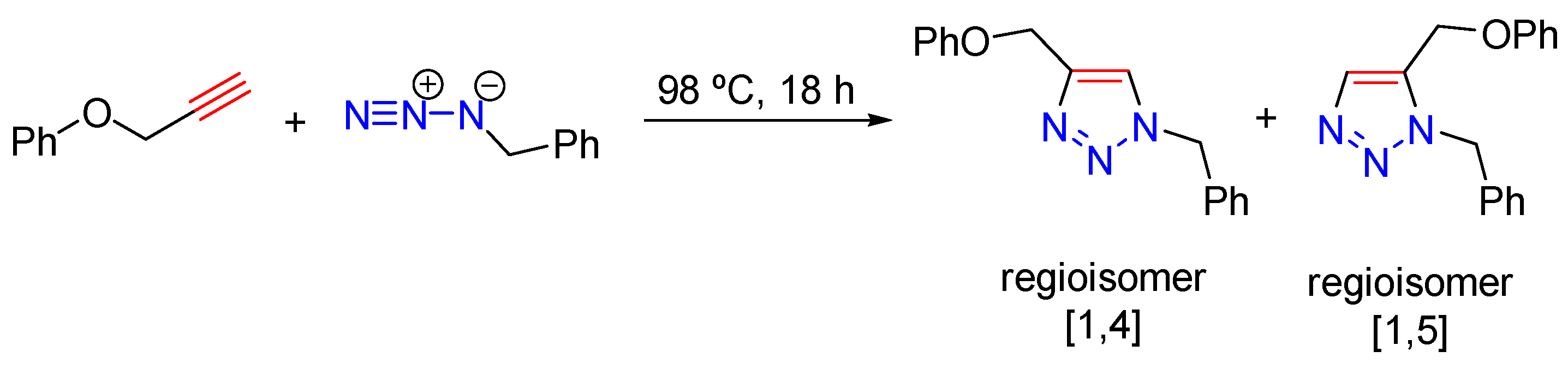
1. Introduction
2. Materials and Methods
2.1. Synthetic Procedures
2.2. General Procedure for the Synthesis of β-Azidophosphonates
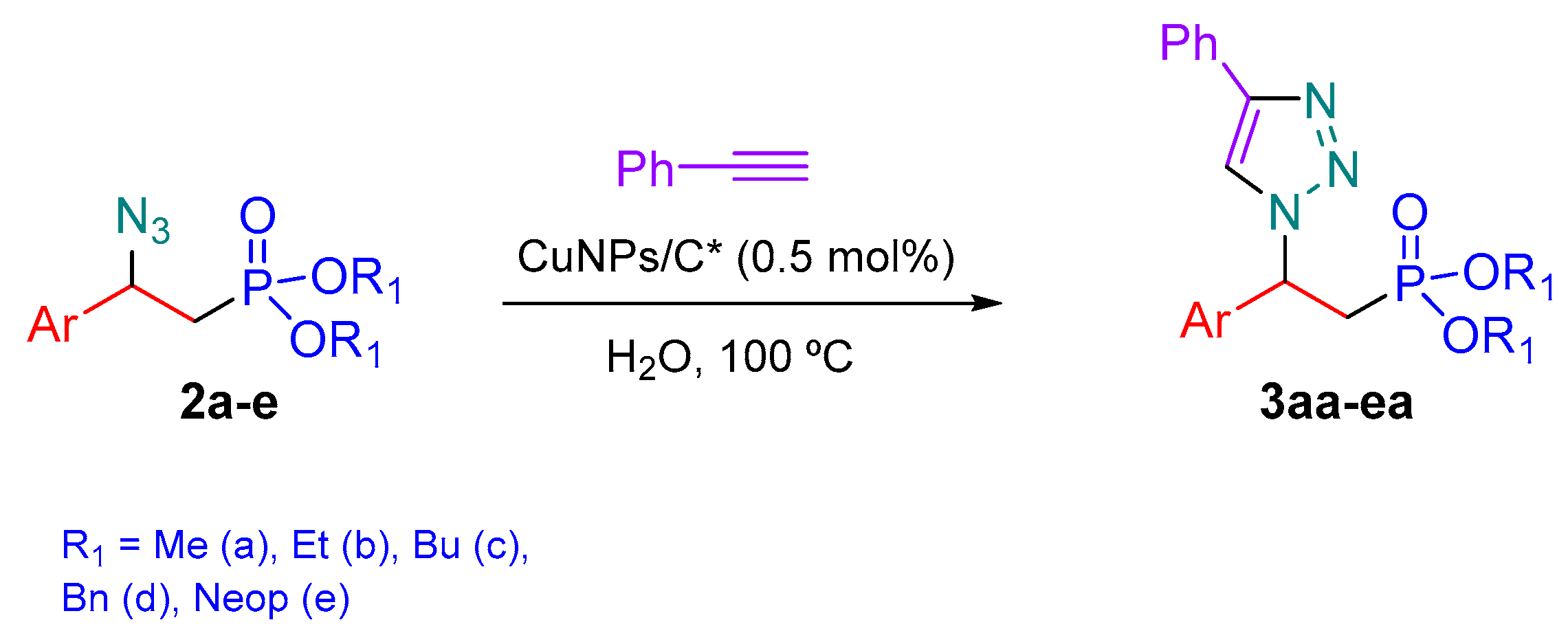
2.3. General Procedure for 1,3-Cycloaddition of β-Azidophosphonates with Alkynes Catalyzed by Copper Nanoparticles Supported on Activate Carbon (CuNPs/C*) in Water
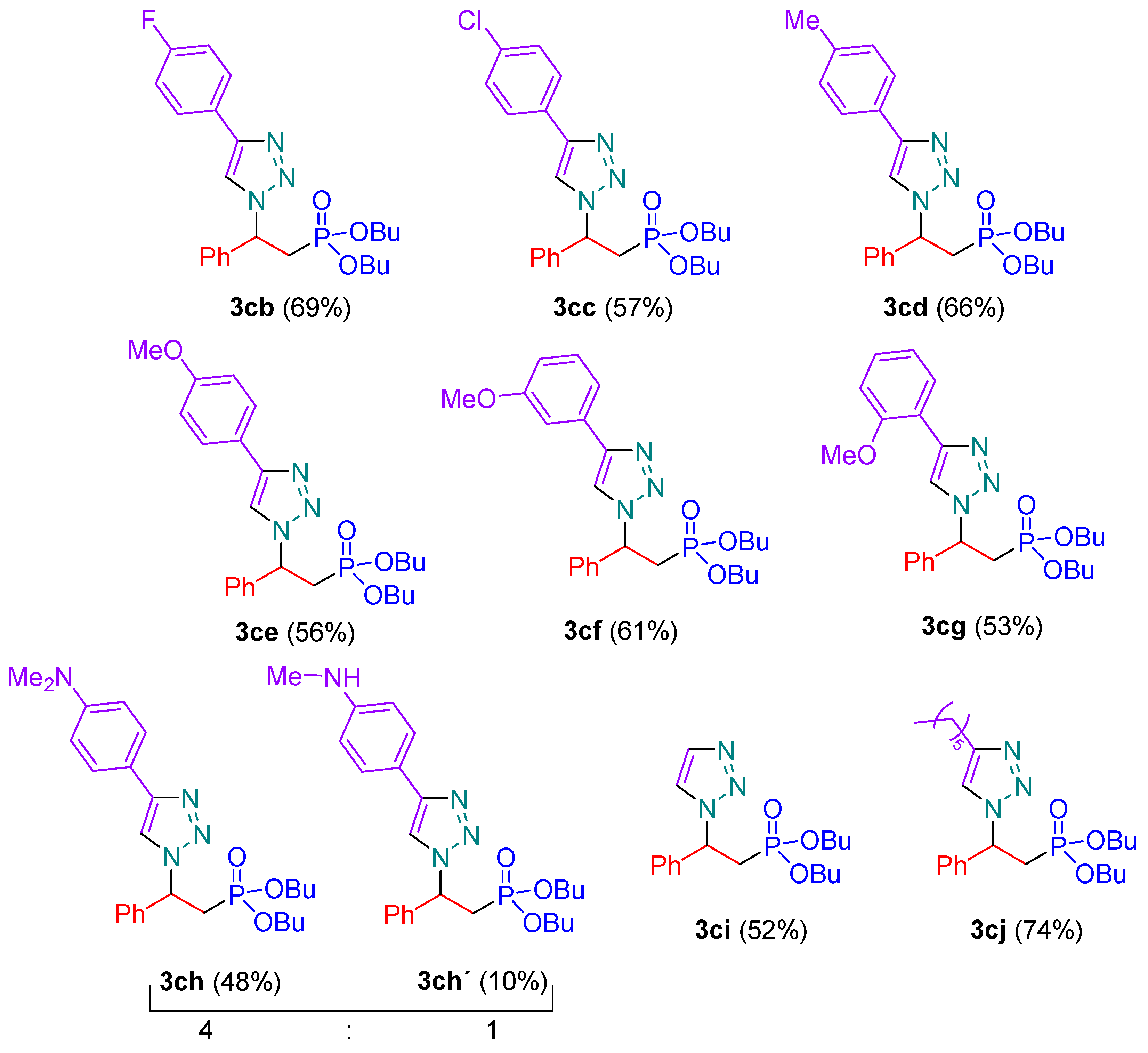
3. Results and Discussion
3.1. Synthesis of β-Azidophosphonates
3.2. Synthesis of 1,2,3-Triazolylphophonates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kerru, N.; Gummidi, L.; Maddila, S.; Gangu, K.K.; Jonnalagadda, S.B. A Review on Recent Advances in Nitrogen-Containing Molecules and Their Biological Applications. Molecules 2020, 25, 1909. [Google Scholar] [CrossRef] [PubMed]
- Bozorov, K.; Zhao, J.; Aisa, H.A. 1,2,3-Triazole-containing hybrids as leads in medicinal chemistry: A recent overview. Bioorg. Med. Chem. 2019, 27, 3511–3531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B. Comprehensive review on the anti-bacterial activity of 1,2,3-triazole. Eur. J. Med. Chem. 2019, 168, 357–372. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhao, S.-J.; Liu, Y. 1,2,3-Triazole-containing hybrids as potential anticancer agents: Current developments, action mechanisms and structure-activity relationships hybrids. Eur. J. Med. Chem. 2019, 183, 111700. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sharma, B.; Mehra, V.; Kumar, V. Recent accomplishments on the synthetic/biological facets of pharmacologically active 1H-1,2,3-triazoles. Eur. J. Med. Chem. 2020, 212, 113069. [Google Scholar] [CrossRef] [PubMed]
- Huisgen, R. Centenary Lecture: 1,3-Dipolar Cycloadditions. Proc. Chem. Soc. 1961, 357–396. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Kolb, H.C.; Sharpless, K.B. The growing impact of click chemistry on drug discovery. Drug Discov. Today 2003, 8, 1128–1137. [Google Scholar]
- Ramapanicker, R.; Chauhan, P. Click Chemistry: Mechanistic and Synthetic Perspectives. In Click Reactions in Organic Synthesis, 1st ed.; Chandrasekaran, S., Ed.; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2016; pp. 1–22. [Google Scholar]
- Alonso, F.; Moglie, F. Copper Nanoparticles in Click Chemistry. Acc. Chem. Res. 2015, 48, 2516–2528. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.B.; Gallo-Rodriguez, C. The Role of the Phosphorus Atom in Drug Design. ChemMedChem 2019, 14, 190–216. [Google Scholar] [CrossRef] [PubMed]
- Demmer, C.S.; Krogsgaar-Larsen, N.; Bunch, L. Review on Modern Advances of Chemical Methods for the Introduction of a Phosphonic Acid Group. Chem. Rev. 2011, 111, 7981–8006. [Google Scholar] [CrossRef]
- Gutierrez, V.; Mascaró, E.; Alonso, F.; Moglie, Y.; Radivoy, G. Direct synthesis of β-ketophosphonates and vinyl phosphonates from alkenes or alkynes catalyzed by CuNPs/ZnO. RSC Adv. 2015, 5, 65739–65744. [Google Scholar] [CrossRef]
- Alonso, F.; Moglie, Y.; Radivoy, G.; Yus, M. Click chemistry from organic halides, diazonium salts and anilines in water catalysed by copper nanoparticles on activated carbon. Org. Biomol. Chem. 2011, 9, 6385–6395. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, V.; Moglie, Y.; Murray, A.P.; Radivoy, G. Alkynyl and β-ketophosphonates: Selective and potent butyrylcholinesterase inhibitors. Bioorg. Chem. 2018, 77, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Moglie, Y.; Buxaderas, E.; Mancini, A.; Alonso, F.; Radivoy, G. Amide Bond Formation Catalyzed by Recyclable Copper Nanoparticles Supported on Zeolite Y under Mild Conditions. ChemCatChem 2019, 11, 1487–1494. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bjerg, E.; Marchán-García, J.; Radivoy, G.; Moglie, Y.; Buxaderas, E. Organophosphorus Chemistry: Synthesis of New Phosphonic Acid Derivatives Bearing a Triazole Moiety. Chem. Proc. 2022, 12, 9. https://doi.org/10.3390/ecsoc-26-13585
Bjerg E, Marchán-García J, Radivoy G, Moglie Y, Buxaderas E. Organophosphorus Chemistry: Synthesis of New Phosphonic Acid Derivatives Bearing a Triazole Moiety. Chemistry Proceedings. 2022; 12(1):9. https://doi.org/10.3390/ecsoc-26-13585
Chicago/Turabian StyleBjerg, Esteban, Joaquín Marchán-García, Gabriel Radivoy, Yanina Moglie, and Eduardo Buxaderas. 2022. "Organophosphorus Chemistry: Synthesis of New Phosphonic Acid Derivatives Bearing a Triazole Moiety" Chemistry Proceedings 12, no. 1: 9. https://doi.org/10.3390/ecsoc-26-13585
APA StyleBjerg, E., Marchán-García, J., Radivoy, G., Moglie, Y., & Buxaderas, E. (2022). Organophosphorus Chemistry: Synthesis of New Phosphonic Acid Derivatives Bearing a Triazole Moiety. Chemistry Proceedings, 12(1), 9. https://doi.org/10.3390/ecsoc-26-13585





