A New Hybrid Molecule Based on (5Z,9Z)-icosa-5,9-dienoic Acid and Monocarbonyl Derivatives of Curcuminoids †
Abstract
1. Introduction
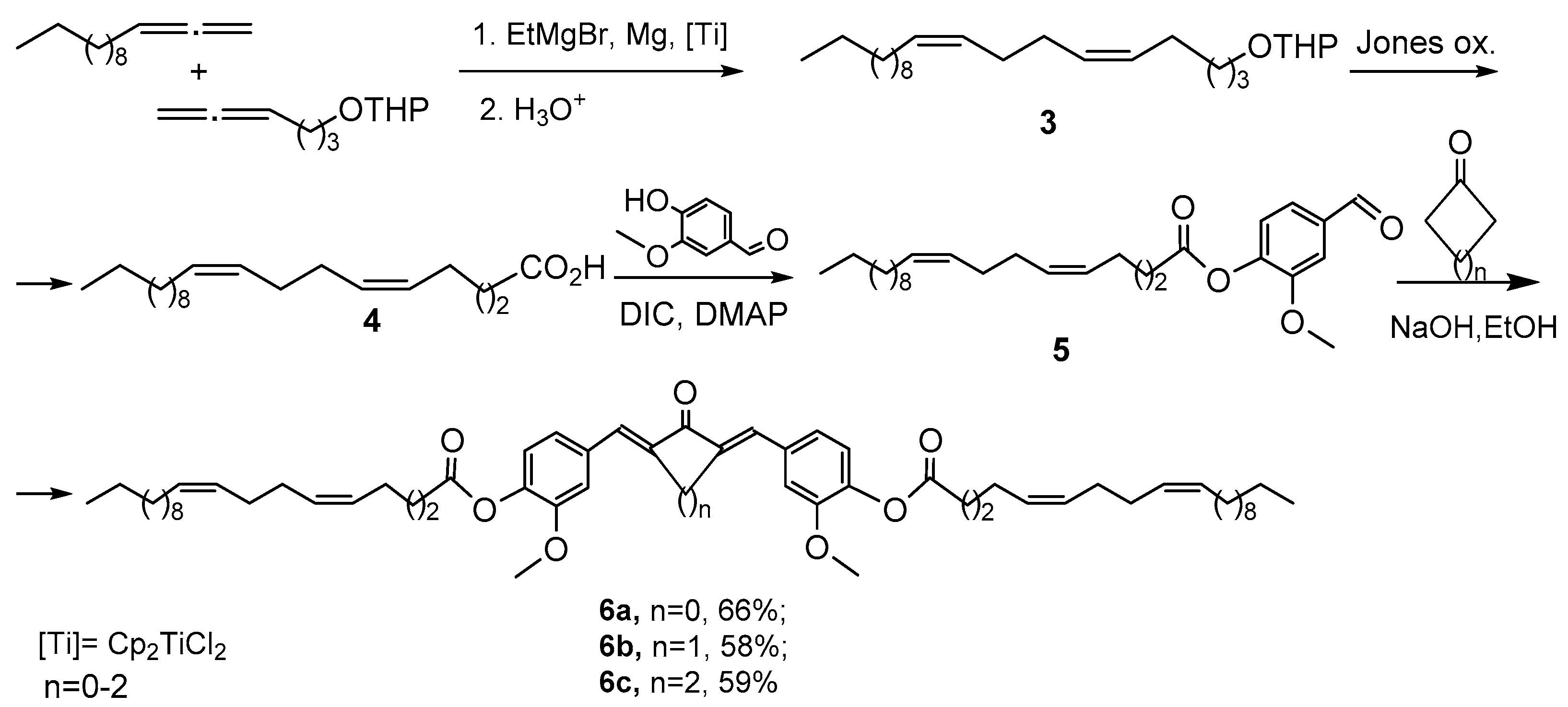
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gediya, L.K.; Njar, V.C.O. Promise and challenges in drug discovery and development of hybrid anticancer drugs. Exp. Opin. Drug Disc. 2009, 4, 1099–1111. [Google Scholar] [CrossRef] [PubMed]
- Fortin, S.; Berube, G. Advances in the development of hybrid anticancer drugs. Exp. Opin. Drug Disc. 2013, 8, 1029–1047. [Google Scholar] [CrossRef] [PubMed]
- Rather, M.A.; Bhat, B.A.; Qurishi, M.A. Multicomponent phytotherapeutic approach gaining momentum: Is the “one drug to fit all” model breaking down? Phytomedicine 2013, 21, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Nepali, K.; Sharma, S.; Kumar, D.; Budhiraja, A.; Dhar, K.L. Anticancer hybrids-a patent survey. Recent Pat. Anticancer Drug Discov. 2014, 9, 303–339. [Google Scholar] [CrossRef]
- Bansal, Y.; Silakari, O. Multifunctional compounds: Smart molecules for multifactorial diseases. Eur. J. Med. Chem. 2014, 76, 31–42. [Google Scholar] [CrossRef]
- Morphy, R.; Kay, C.; Rankovic, Z. From magic bullets to designed multiple ligands. Drug Discov. Today 2004, 9, 641–651. [Google Scholar] [CrossRef]
- Nepali, K.; Sharma, S.; Sharma, M.; Bedi, P.M.S.; Dhar, K.L. Rational approaches, design strategies, structure activity relationship and mechanistic insights for anticancer hybrids. Eur. J. Med. Chem. 2014, 77, 422–487. [Google Scholar] [CrossRef]
- Liang, G.; Shao, L.; Wang, Y.; Zhao, C.; Chu, Y.; Xiao, J.; Zhao, Y.; Li, X.; Yang, S. Exploration and synthesis of curcumin analogues with improved structural stability both in vitro and in vivo as cytotoxic agents. Bioorg. Med. Chem. 2009, 17, 2623–2631. [Google Scholar] [CrossRef]
- Sanabria-Rios, D.J.; Rivera-Torres, Y.; Rosario, J.; Gutierrez, R.; Torres-García, Y.; Montano, N.; Ortíz-Soto, G.; Rios-Olivares, E.; Rodriguez, J.W.; Carballeira, N.M. Chemical conjugation of 2-hexadecynoic acid to C5-curcumin enhances its antibacterial activity against multi-drug resistant bacteria. Bioorg. Med. Chem. Lett. 2015, 25, 5067–5071. [Google Scholar] [CrossRef]
- Leow, P.C.; Bahety, P.; Boon, C.P.; Lee, C.Y.; Tan, K.L.; Yang, T.; Ee, P.L. Functionalized curcumin analogs as potent modulators of the Wnt/β-catenin signaling pathway. Eur. J. Med. Chem. 2014, 71, 67–80. [Google Scholar] [CrossRef]
- Pan, Z.; Chen, C.; Zhou, Y.; Xu, F.; Xu, Y. Synthesis and Cytotoxic Evaluation of Monocarbonyl Analogs of Curcumin as Potential Anti-Tumor Agents. Drug Dev. Res. 2016, 77, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Kohyama, A.; Yamakoshi, H.; Hongo, S.; Kanoh, N.; Shibata, H.; Iwabuchi, Y. Structure-Activity Relationships of the Antitumor C5-Curcuminoid GO-Y030. Molecules 2015, 20, 15374–15391. [Google Scholar] [CrossRef] [PubMed]
- Teiten, M.H.; Dicato, M.; Diederich, M. Hybrid curcumin compounds: A new strategy for cancer treatment. Molecules 2014, 19, 20839–20863. [Google Scholar] [CrossRef] [PubMed]
- Noureddin, S.A.; El-Shishtawy, R.M.; Al-Footy, K.O. Curcumin analogues and their hybrid molecules as multifunctional drugs. Eur. J. Med. Chem. 2019, 182, 111631. [Google Scholar] [CrossRef]
- Dias, K.S.T.; de Paula, C.T.; dos Santos, T.; Souza, I.N.O.; Boni, M.S.; Guimarães, M.J.R.; da Silva, F.M.R.; Castro, N.G.; Neves, G.; Veloso, C.C.; et al. Design, synthesis and evaluation of novel feruloyl-donepezil hybrids as potential multitarget drugs for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2017, 130, 440–457. [Google Scholar] [CrossRef]
- Sharma, S.; Gupta, M.K.; Saxena, A.K.; Bedi, P.M.S. Triazole linked mono carbonyl curcumin-isatin bifunctional hybrids as novel anti tubulin agents: Design, synthesis, biological evaluation and molecular modeling studies. Bioorg. Med. Chem. 2015, 23, 7165–7180. [Google Scholar] [CrossRef]
- Allegra, A.; Innao, V.; Russo, S.; Gerace, D.; Alonci, A.; Musolino, C. Anticancer Activity of Curcumin and Its Analogues: Preclinical and Clinical Studies. Cancer Investig. 2017, 35, 1–22. [Google Scholar] [CrossRef]
- Singh, A.; Singh, J.V.; Rana, A.; Bhagat, K.; Gulati, H.K.; Kumar, R.; Salwan, R.; Bhagat, K.; Kaur, G.; Singh, N.; et al. Monocarbonyl Curcumin-Based Molecular Hybrids as Potent Antibacterial Agents. ACS Omega 2019, 4, 11673–11684. [Google Scholar] [CrossRef]
- Pommier, Y. DNA Topoisomerase I Inhibitors: Chemistry, Biology, and Interfacial Inhibition. Chem. Rev. 2009, 109, 2894. [Google Scholar] [CrossRef]
- Carballeira, N.M.; Montano, N.; Amador, L.A.; Rodríguez, A.D.; Golovko, M.Y.; Golovko, S.A.; Reguera, R.M.; Álvarez-Velilla, R.; Balaña-Fouce, R. Novel Very Long-Chain α-Methoxylated Δ5,9 Fatty Acids from the Sponge Asteropus niger Are Effective Inhibitors of Topoisomerases IB. Lipids 2016, 51, 245. [Google Scholar] [CrossRef]
- Makarieva, T.N.; Santalova, E.A.; Gorshkova, I.A.; Dmitrenok, A.S.; Guzii, A.G.; Gorbach, V.I.; Svetashev, V.I.; Stonik, V.A. A new cytotoxic fatty acid (5Z,9Z)-22-methyl-5,9-tetracosadienoic acid and the sterols from the far eastern sponge Geodinella robusta. Lipids 2002, 37, 75. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Dzhemileva, L.U.; Dzhemilev, U.M. Natural compounds with bis-methylene-interrupted Z-double bonds: Plant sources, strategies of total synthesis, biological activity, and perspectives. Phytochem. Rev. 2021, 20, 325–342. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Dzhemileva, L.U.; Makarov, A.A.; Makarova, E.K.; Khusnutdinova, E.K.; Dzhemilev, U.M. The facile synthesis of the 5Z,9Z-dienoic acids and their topoisomerase I inhibitory activity. Chem. Commun. 2013, 49, 8401–8403. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Islamov, I.I.; Dzhemileva, L.U.; Khusainova, E.M.; Yunusbaeva, M.M.; Dzhemilev, U.M. Targeted synthesis of macrodiolides containing bis-methylene-separated Z-double bonds and their antitumor activity in vitro. Tetrahedron 2018, 74, 4606–4612. [Google Scholar] [CrossRef]
- Dzhemileva, L.U.; D’yakonov, V.A.; Islamov, I.I.; Yunusbaeva, M.M.; Dzhemilev, U.M. New 1Z,5Z-diene macrodiolides: Catalytic synthesis, anticancer activity, induction of mitochondrial apoptosis, and effect on the cell cycle. Bioorg. Chem. 2020, 99, 103832. [Google Scholar] [CrossRef] [PubMed]
- D’yakonov, V.A.; Dzhemileva, L.U.; Makarov, A.A.; Mulukova, A.R.; Baev, D.S.; Khusnutdinova, E.K.; Tolstikova, T.G.; Dzhemilev, U.M. nZ,(n + 4)Z-Dienoic fatty acids: A new method for the synthesis and inhibitory action on topoisomerase I and Iiα. Med. Chem. Res. 2016, 25, 30–39. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Dzhemileva, L.U.; Tuktarova, R.A.; Makarov, A.A.; Islamov, I.I.; Mulukova, A.R.; Dzhemilev, U.M. Catalytic cyclometallation in steroid chemistry III: Synthesis of steroidal derivatives of 5Z, 9Z-dienoic acid and investigation of its human topoisomerase I inhibitory activity. Steroids 2015, 102, 110–117. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Islamov, I.I.; Dzhemileva, L.U.; Makarova, E.K.; Dzhemilev, U.M. Direct synthesis of polyaromatic cyclophanes containing bis-methylene-interrupted Z-double bonds and study of their antitumor activity in vitro. Int. J. Mol. Sci. 2021, 22, 8787. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islamov, I.; Yusupova, A.; Sharafutdinova, S.; Dzhemilev, U. A New Hybrid Molecule Based on (5Z,9Z)-icosa-5,9-dienoic Acid and Monocarbonyl Derivatives of Curcuminoids. Chem. Proc. 2022, 12, 45. https://doi.org/10.3390/ecsoc-26-13636
Islamov I, Yusupova A, Sharafutdinova S, Dzhemilev U. A New Hybrid Molecule Based on (5Z,9Z)-icosa-5,9-dienoic Acid and Monocarbonyl Derivatives of Curcuminoids. Chemistry Proceedings. 2022; 12(1):45. https://doi.org/10.3390/ecsoc-26-13636
Chicago/Turabian StyleIslamov, Ilgiz, Adelya Yusupova, Snezhana Sharafutdinova, and Usein Dzhemilev. 2022. "A New Hybrid Molecule Based on (5Z,9Z)-icosa-5,9-dienoic Acid and Monocarbonyl Derivatives of Curcuminoids" Chemistry Proceedings 12, no. 1: 45. https://doi.org/10.3390/ecsoc-26-13636
APA StyleIslamov, I., Yusupova, A., Sharafutdinova, S., & Dzhemilev, U. (2022). A New Hybrid Molecule Based on (5Z,9Z)-icosa-5,9-dienoic Acid and Monocarbonyl Derivatives of Curcuminoids. Chemistry Proceedings, 12(1), 45. https://doi.org/10.3390/ecsoc-26-13636





