Abstract
Organophosphorus compounds have been investigated for agricultural and medicinal applications for decades, and a considerable number of phosphorus-containing drugs have achieved commercial success. A recent review by P. Finkbeiner et al. has shown that phosphine oxides and related phosphorus-containing functional groups are valuable polar structural elements and that they deserve to be considered as a routine part of every medicinal chemist’s toolbox. A new approach to the synthesis of previously hard-to-obtain 3-alkyl-1H-phospholanes oxides was developed by us. In order to assess the potential of five-membered cyclic organophosphorus compounds in cancer therapy, we carried out docking 3-buthyl-1H-phospholanes oxide and 2,3-dihydrophosphole in the binding site of 24 human proteins involved in oncogenesis processes. Proteins were selected using the PharmMapper in-house pharmacophore model database. The results are presented in the article.
1. Introduction
It is well known that organophosphorus compounds are used in medicine, moreover, a significant number of phosphorus-containing drugs have achieved commercial success [1]. Recently, a review by P. Finkbeiner has shown [2] that most of the approved phosphorus-containing pharmaceuticals, for example drugs 1–6, contain a phosphate, a phosphoramide, or a phosphonate group, while phosphines, phosphinates, and phosphine oxides are rare (Scheme 1). For example, the phosphinate-based drug used to treat hypertension is fosinopril (7). Recently, ridaforolimus (12), a dimethylphosphinic ester containing inhibitor of mammalian target of rapamycin (mTOR), progressed into phase III clinical studies for the treatment of sarcoma, and the anaplastic lymphoma kinase (ALK) inhibitor brigatinib (13) became the first drug containing a phosphine oxide motif that was approved for the treatment of patients with metastatic non-small-cell lung cancer (NSCLC) [3,4].
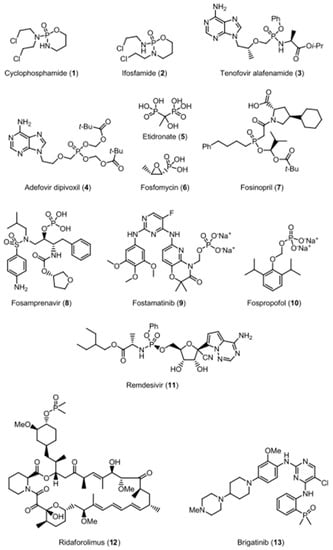
Scheme 1.
Selected examples of phosphorus-containing drugs.
At the same time, new approaches to the synthesis of previously undescribed cyclic phospholane oxides are being developed.
We have accumulated significant experience in the development of effective one-pot methods for the synthesis of five-membered phosphacarbocycles via transmetalation of aluminacarbocycles, obtained by catalytic cycloalumination [5,6,7] of olefins with AlEt3 in the presence of Cp2ZrCl2 as a catalyst, by PCl3 (Scheme 2).
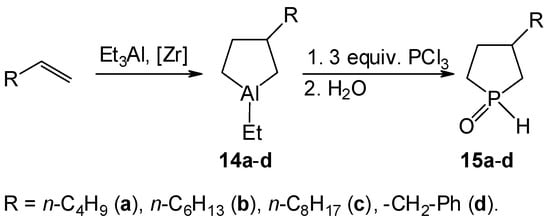
Scheme 2.
Synthesis of the 3-alkyl(aryl)-1H-phospholane oxides via transmetalation of alumolanes by PCl3.
The synthesized compounds are chemically stable and may be promising in cancer therapy. In order to predict the biological properties for oncotherapy of a number of phospholane oxides, we screened using the PharmMapper. Then docking was employed using AutoDock to find out the mechanism of binding of the macromolecular targets to small active components under consideration, which made it possible to determine the role of the P=O(H) group in the interaction with targets.
2. Methods
A search for potential protein targets for the studied ligands was carried out using the PharmMapper in-house pharmacophore model database [8]. For this, the optimized ligand structures were saved as SDF files, which were then uploaded to a web server available at http://www.lilab-ecust.cn/pharmmapper/ (accessed on 22 October 2022). Pharmacophore mapping was carried out for the human protein targets set. From the resulting list of the potential human protein targets, only those involved in the processes of oncogenesis were selected for further study. AutoDock Tools (ADT) version 4.2.6 was used to carry out protein-ligand docking simulations [9]. The Discovery Studio Visualizer (version 21.1.0.20298, Dassault Systèmes, San Diego, CA, USA) [10] software was used to visualize the docking results.
3. Results and Discussion
The potential human protein targets were identified for model compound 15a. Two diastereomers were taken into consideration with energy energetically lowest twist conformation (Scheme 3). The screening results showed 17 ranked targets listed in Table 1, confirming the possibility of interaction between the model compound and some indications. The highest fit scores for both isomers was characterized the androgen receptor, which is a member of the steroid/nuclear receptor superfamily and which functions as a transcription factor [11]. This receptor is activated by binding to androgenic hormones that regulate male sex development [12]. Reactivation of the androgen receptor occurs in recurrent prostate cancer [13], making this protein a potential target for prostate cancer therapy.
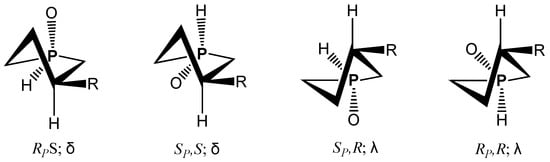
Scheme 3.
Diastereomers of phospholane.

Table 1.
Potential targets and indications of compound 2a (RR configuration) by PharmMapper.
The receptor was selected for the molecular docking simulation (Figure 1). Accordingly, the bioactive molecule in lowest conformation forms intermolecular interactions between the P=O group and the residues. The active sites of binding region in the receptor for the co-crystallized structure, which taken for comparison, were differ.
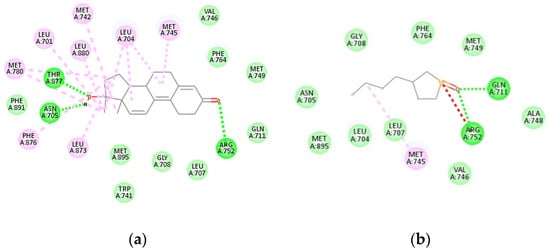
Figure 1.
2-D diagram showing the intermolecular interactions with the active site residues of the androgen receptor of (a) co-crystallized ligand and (b) RR phospholane. Hydrophobic interactions are colored in light pink, unfavorable positive-positive interaction is colored in red, van der Waals interactions is colored in mint green, conventional hydrogen bonding is colored in green.
An free binding energy and final intermolecular energy, as well as an inhibition constant for each of the docked bioactive molecules, were estimated (Table 2). In terms of inhibitory activity, phospholane is clearly lower to the co-crystalized ligand (FBE = −10.04 kcal/mol, Ki = 43.69 nM).

Table 2.
The lowest energy docked conformation of studied phospholanes.
In the case of RR phospholane interaction with the active site of the androgen receptor, the hydrogen bonds were formed with the P=O functional group. Out of the total interactions, there was a lack of hydrophobic contacts, obviously; therefore, with an increase in chain length of the alkyl substituent, an increase in the binding energy was observed. It should be noted that the effect of stereochemistry on the energy parameters was also manifested. Moreover, we have docked the tautomeric form P-OH [14], which can exist at the equilibrium concentration (denoted as 15′) known for phosphine oxides (Table 2).
4. Conclusions
In summary, the potential anticancer activity for new 1H-phospolane oxides was identified. The androgen receptor was selected for the molecular docking simulation, as a result a binding site between the P=O and protein was shown. It was found that the design of the substituent in position 3 helped to model the binding activity.
Author Contributions
Conceptualization, T.V.T.; methodology, validation, and execution of chemistry experiments, D.N.I., M.I.M., and A.L.M.; manuscript preparation, M.I.M. and T.V.T. All authors have read and agreed to the published version of the manuscript.
Funding
The work was carried out within approved plans for research projects at the IPC RAS State Registration No. FMRS-2022-0074.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request.
Acknowledgments
The structural studies of the synthesized compounds were performed on the equipment of «Agidel» Collective Usage Center of Ufa Federal Research Center at the Institute of Petrochemistry and Catalysis, Ufa Federal Research Center, Russian Academy of Sciences.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Finkbeiner, P.; Hehn, J.P.; Gnamm, C. Phosphine Oxides from a Medicinal Chemist’s Perspective: Physicochemical and In Vitro Parameters Relevant for Drug Discovery. J. Med. Chem. 2020, 63, 7081–7107. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.B.; Gallo-Rodriguez, C. The Role of the Phosphorous Atom in Drug Design. Chem. Med. Chem. 2019, 14, 190–216. [Google Scholar] [PubMed]
- Markham, A. Brigatinib: First Global Approval. Drugs 2017, 77, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.S.; Liu, S.; Zou, D.; Thomas, M.; Wang, Y.; Zhou, T.; Romero, J.; Kohlmann, A.; Li, F.; Qi, J.; et al. Discovery of Brigatinib (AP26113), a Phosphine Oxide-Containing, Potent, Orally Active Inhibitor of Anaplastic Lymphoma Kinase. J. Med. Chem. 2016, 59, 4948–4964. [Google Scholar] [CrossRef] [PubMed]
- Ibragimov, A.G.; Khafizova, L.O.; Khusainova, L.I.; Tyumkina, T.V.; Dzhemilev, U.M. Joint cycloalumination of ethylene and other unsaturated compounds with EtAlCl2 in the presence of Cp2ZrCl2. Synthesis of aluminacarbocycles. Russ. J. Org. Chem. 2010, 46, 474–479. [Google Scholar] [CrossRef]
- Dzhemilev, U.M.; Ibragimov, A.G. Catalytic cyclometalation reaction of unsaturated compounds in synthesis of magnesa- and aluminacarbocycles. J. Organomet. Chem. 2010, 695, 1085–1110. [Google Scholar] [CrossRef]
- Khafizova, L.O.; Khusainova, L.I.; Tyumkina, T.V.; Dzhemilev, U.M. One-pot synthesis of borolanes by reaction of aluminacyclopentanes with BF3·Et2O. Russ. J. Org. Chem. 2012, 48, 755–760. [Google Scholar] [CrossRef]
- Wang, X.; Shen, Y.; Wang, S.; Li, S.; Zhang, W.; Liu, X.; Lai, L.; Pei, J.; Li, H. PharmMapper 2017 update: A web server for potential drug target identification with a comprehensive target pharmacophore database. Nucleic Acids Res. 2017, 45, W356–W360. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- BIOVIA Dassault Systèmes. Discovery Studio Visualizer; v21.1.0.20298; Dassault Systèmes: San Diego, CA, USA, 2020. [Google Scholar]
- Gregory, C.W.; Hamil, K.G.; Kim, D.; Hall, S.H.; Pretlow, T.G.; Mohler, J.L.; French, F.S. Androgen receptor expression in androgen-independent prostate cancer is associated with increased expression of androgen regulated genes. Cancer Res. 1998, 58, 5718–5724. [Google Scholar] [PubMed]
- Quigley, C.A.; de Bellis, A.; Marschke, K.B.; El-Awady, M.K.; Wilson, E.M.; French, F.S. Androgen Receptor Defects: Historical, Clinical, and Molecular Perspectives. Endocr. Rev. 1995, 16, 271–321. [Google Scholar] [CrossRef] [PubMed]
- Gregory, C.W.; He, B.; Johnson, R.T.; Ford, O.H.; Mohler, J.L.; French, F.S.; Wilson, E.M. A mechanism for androgen receptor mediated prostate cancer recurrence after androgen deprivation therapy. Cancer Res. 2001, 61, 4315–4319. [Google Scholar] [PubMed]
- Schlemminger, I.; Saida, Y.; Groger, H.; Maison, W.; Durot, N.; Sasai, H.; Shibasaki, M.; Martens, J. Concept of Improved Rigidity: How to Make Enantioselective Hydrophosphonylation of Cyclic Imines Catalyzed by Chiral Heterobimetallic Lanthanoid Complexes Almost Perfect. J. Org. Chem. 2000, 65, 4818–4825. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).