A Facile Method for Assessing the Change in Detonation Properties during Chemical Functionalization: The Case of NH2→NHNO2 and NH2→=N+=N− Conversions †
Abstract
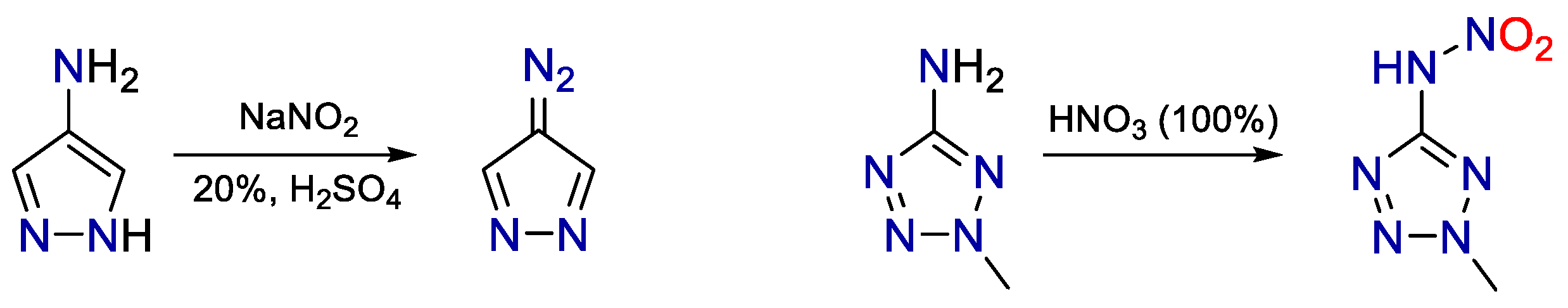
1. Introduction
2. Computational Method
3. Results and Discussion
3.1. Calculation at Level 1
3.2. Calculations at Level 2
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bondarchuk, S.V. Magic of numbers: A guide for preliminary estimation of the detonation performance of C–H–N–O explosives based on empirical formulas. Ind. Eng. Chem. Res. 2021, 60, 1952–1961. [Google Scholar] [CrossRef]
- Bondarchuk, S.V. Diazoamination: A simple way to enhance detonation performance of aminoaromatic and aminoheterocyclic energetic materials. FirePhysChem 2021, 1, 97–102. [Google Scholar] [CrossRef]
- Bondarchuk, S.V. Structure enhancement of energetic materials: A theoretical study of the arylamines to arylpentazoles transformation. FirePhysChem 2021, 1, 190–197. [Google Scholar] [CrossRef]
- Tang, J.; Yang, H.; Cui, Y.; Cheng, G. Nitrogen-rich tricyclic-based energetic materials. Mater. Chem. Front. 2021, 5, 7108–7118. [Google Scholar] [CrossRef]
- Wang, L.; Zhai, L.; She, W.; Wang, M.; Zhang, J.; Wang, B. Synthetic strategies toward nitrogen-rich energetic compounds via the reaction characteristics of cyanofurazan/furoxan. Front. Chem. 2022, 10, 871684. [Google Scholar] [CrossRef]
- Luo, Y.; Zheng, W.; Wang, X.; Shen, F. Nitrification progress of nitrogen-rich heterocyclic energetic compounds: A review. Molecules 2022, 27, 1465. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Adeniyi, A.O. Solid nitrogen and nitrogen-rich compounds as high-energy-density materials. Phys. Status Solidi B 2021, 258, 2000588. [Google Scholar] [CrossRef]
- Yang, J.; Wang, G.; Gong, X.; Zhang, J.; Wang, Y.A. High-energy nitramine explosives: A design strategy from linear to cyclic to caged molecules. ACS Omega 2018, 3, 9739–9745. [Google Scholar] [CrossRef] [PubMed]
- Green, S.P.; Wheelhouse, K.M.; Payne, A.D.; Hallett, J.P.; Miller, P.W.; Bull, J.A. Thermal stability and explosive hazard assessment of diazo compounds and diazo transfer reagents. Org. Process Res. Dev. 2019, 24, 67–84. [Google Scholar] [CrossRef]
- Antonsen, S.; Aursnes, M.; Gallantree-Smith, H.; Dye, C.; Stenstrøm, Y. Safe synthesis of alkylhydroxy and alkylamino nitramines. Molecules 2016, 21, 1738. [Google Scholar] [CrossRef]
- Zollinger, H.B. Diazo Chemistry II Aliphatic, Inorganic and Organometallic Compounds; VCH: Weinheim, Germany, 1995; pp. 11–95. [Google Scholar]
- Buckle, D.R.; Pinto, I.L. Functions bearing two nitrogens. In Comprehensive Organic Functional Group Transformations; Elsevier: Amsterdam, The Netherlands, 1995; Volume 4, pp. 403–449. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, J.; Peng, P.; Su, H.; Li, S.; Pang, S. Synthesis and characterization of three pyrazolate inner diazonium salts: Green, powerful and stable primary explosives. New J. Chem. 2017, 41, 9244–9249. [Google Scholar] [CrossRef]
- Klapötke, T.M. New nitrogen-rich high explosives. Struct. Bond. 2007, 125, 85–121. [Google Scholar] [CrossRef]
- Dalinger, I.L.; Cherkasova, T.I.; Shevelev, S.A. Synthesis of 4-diazo-3,5-dinitropyrazole and characteristic features of its behaviour towards nucleophiles. Mendeleev Commun. 1997, 7, 58–59. [Google Scholar] [CrossRef]
- Materials Studio 2017; Dassault Systèmes BIOVIA: San Diego, CA, USA, 2016.
- Sun, H.; Jin, Z.; Yang, C.; Akkermans, R.L.C.; Robertson, S.H.; Spenley, N.A.; Miller, S.; Todd, S.M. COMPASS II: Extended coverage for polymer and drug-like molecule databases. J. Mol. Model. 2016, 22, 47. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Delley, B. From molecules to solids with the DMol3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Jacobs, S.J. Chemistry of detonations. I. A simple method for calculating detonation properties of C–H–N–O explosives. J. Chem. Phys. 1968, 48, 23–35. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, Q.; Shreeve, J.M. Fused heterocycle-based energetic materials (2012–2019). J. Mater. Chem. A 2020, 8, 4193–4216. [Google Scholar] [CrossRef]
- Gao, H.; Shreeve, J.M. Azole-based energetic salts. Chem. Rev. 2011, 111, 7377–7436. [Google Scholar] [CrossRef]
- Yin, P.; Shreeve, J.M. Nitrogen-rich azoles as high density energy materials. Adv. Heterocycl. Chem. 2017, 121, 89–131. [Google Scholar] [CrossRef]
- Bennion, J.C.; McBain, A.; Son, S.F.; Matzger, A.J. Design and synthesis of a series of nitrogen-rich energetic cocrystals of 5,5′-dinitro-2H,2h′-3,3′-bi-1,2,4-triazole (DNBT). Crystal Growth Des. 2015, 15, 2545–2549. [Google Scholar] [CrossRef]
- Yount, J.; Zeller, M.; Byrd, E.F.C.; Piercey, D.G. 4,4′-Dinitrimino-5,5′-diamino-3,3′-azo-bis-1,2,4-triazole: A high-performing zwitterionic energetic material. Inorg. Chem. 2021, 60, 16204–16212. [Google Scholar] [CrossRef] [PubMed]
- Chinnam, A.K.; Staples, R.J.; Shreeve, J.M. Nucleophilic catalyzed structural binary cleavage of a fused [5,5]-bicyclic compound. Org. Lett. 2021, 23, 9408–9412. [Google Scholar] [CrossRef]
- Song, H.; Li, B.; Gao, X.; Shan, F.; Ma, X.; Tian, X.; Chen, X. Thermodynamics and catalytic properties of two novel energetic complexes based on 3-amino-1,2,4-triazole-5-carboxylic acid. ACS Omega 2022, 7, 3024–3029. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Ma, Q.; Zhang, Z.-Q.; Geng, W.; Huang, J.; Yang, W.; Fan, G.-J.; Wang, S. Energetic 1H-imidazo[4,5-d]pyridazine-2,4,7-triamine: A novel nitrogen-rich fused heterocyclic cation with high density. Cryst. Growth Des. 2020, 20, 3406–3412. [Google Scholar] [CrossRef]
- Tang, Y.; He, C.; Mitchell, L.A.; Parrish, D.A.; Shreeve, J.M. Energetic compounds consisting of 1,2,5- and 1,3,4-oxadiazole rings. J. Mater. Chem. A 2015, 3, 23143–23148. [Google Scholar] [CrossRef]
- Mathpati, R.S.; Yadav, A.K.; Ghule, V.D.; Dharavath, S. Potential energetic salts of 5,5′-methylenedi(4H-1,2,4-triazole-3,4-diamine) cation: Synthesis, characterization and detonation performance. Energ. Mater. Front. 2022, 3, 90–96. [Google Scholar] [CrossRef]
- Tang, Y.; An, Z.; Chinnam, A.K.; Staples, R.J.; Shreeve, J.M. Very thermostable energetic materials based on a fused-triazole: 3,6-diamino-1H-[1,2,4]triazolo[4,3-b][1,2,4]triazole. New J. Chem. 2021, 45, 85–91. [Google Scholar] [CrossRef]

| Compounds | a1 | a2 | a3 | a4 | b |
|---|---|---|---|---|---|
| Nitramines | −3885 | −1925 | −2065 | 70 | 40 |
| Diazo compounds | −1939 | −553 | −1659 | 1302 | −2 |
| Entry | Ref. | Level 1 | Level 2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q | D | P | ΔQ | ΔD | ΔP | Q | D | P | ΔQ | ΔD | ΔP | ||
| 1 | [21] | 1345.4 | 7961 | 26.8 | 363.2 | 713 | 5.9 | 1115.5 | 8154 | 30.2 | 555.3 | 1164 | 8.8 |
| 2 | [21] | 1535.1 | 8806 | 34.8 | 80.0 | 537 | 5.0 | 1376.6 | 9371 | 42.3 | 341.4 | 1009 | 9.3 |
| 3 | [21] | 1495.7 | 8668 | 33.4 | 239.3 | 800 | 7.4 | 1534.7 | 9309 | 40.3 | 472.9 | 1096 | 9.7 |
| 4 | [21] | 1525.4 | 8949 | 36.1 | 136.0 | 851 | 7.9 | 1574.3 | 9900 | 47.5 | 364.0 | 1495 | 15.2 |
| 5 | [21] | 1550.6 | 9198 | 38.7 | 116.5 | 741 | 7.3 | 1769.0 | 10,208 | 50.2 | 414.5 | 1580 | 16.6 |
| 6 | [21] | 1535.1 | 8996 | 36.9 | 103.6 | 672 | 6.3 | 1580.5 | 9955 | 48.6 | 236.1 | 1371 | 14.8 |
| 7 | [21] | 1446.7 | 8344 | 30.4 | 209.7 | 716 | 6.2 | 1227.5 | 8779 | 36.3 | 341.8 | 1358 | 12.2 |
| 8 | [21] | 1516.4 | 8431 | 31.3 | 180.4 | 644 | 5.8 | 1419.5 | 8429 | 31.7 | 908.0 | 2028 | 13.8 |
| 9 | [21] | 1393.5 | 8008 | 27.8 | 313.9 | 864 | 7.0 | 1366.1 | 8754 | 36.0 | 505.3 | 1402 | 12.2 |
| 10 | [21] | 1530.6 | 8882 | 36.3 | 76.7 | 517 | 4.9 | 1597.5 | 9373 | 41.8 | 209.8 | 432 | 3.6 |
| 11 | [21] | 1522.7 | 8834 | 35.6 | 93.1 | 615 | 5.7 | 1476.9 | 9209 | 40.2 | 239.6 | 604 | 5.2 |
| 12 | [21] | 1541.8 | 9028 | 37.5 | 83.5 | 556 | 5.3 | 1545.5 | 9298 | 40.7 | 207.0 | 815 | 7.8 |
| 13 | [21] | 1445.5 | 8050 | 28.7 | 119.1 | 769 | 6.1 | 1404.4 | 8162 | 30.0 | 333.5 | 763 | 5.2 |
| 14 | [21] | 1492.8 | 8503 | 32.7 | 94.1 | 624 | 5.5 | 1638.3 | 9526 | 44.0 | 304.5 | 1252 | 12.4 |
| 15 | [21] | 1288.5 | 7589 | 23.8 | 281.1 | 575 | 4.4 | 995.1 | 8142 | 30.8 | 455.6 | 1489 | 11.5 |
| 16 | [22] | 1301.2 | 8096 | 28.6 | 434.6 | 633 | 5.9 | 1476.0 | 7948 | 26.4 | 1036.3 | 1520 | 9.3 |
| 17 | [23] | 1434.1 | 8600 | 33.8 | 111.9 | 422 | 4.3 | 1604.0 | 8524 | 32.2 | 411.9 | 133 | −0.3 |
| 18 | [23] | 1434.1 | 8600 | 33.8 | 111.9 | 422 | 4.3 | 1670.1 | 8765 | 34.6 | 330.5 | −16 | −1.4 |
| 19 | [23] | 1046.7 | 7031 | 20.6 | 473.0 | 879 | 5.9 | 1192.8 | 6741 | 18.2 | 1016.0 | 2069 | 9.6 |
| 20 | [23] | 1079.7 | 7358 | 22.4 | 397.7 | 728 | 5.3 | 945.5 | 6561 | 16.4 | 870.0 | 2638 | 10.3 |
| 21 | [24] | 1388.5 | 8425 | 32.0 | 153.8 | 573 | 5.5 | 1460.0 | 8085 | 28.1 | 522.3 | 506 | 2.7 |
| 22 | [25] | 1574.3 | 8666 | 33.1 | 56.7 | 227 | 2.3 | 1628.7 | 8818 | 34.6 | 251.6 | 270 | 2.0 |
| 23 | [26] | 1316.9 | 8002 | 28.5 | 143.2 | 517 | 4.6 | 1276.1 | 7672 | 25.4 | 479.9 | 620 | 3.5 |
| 24 | [27] | 1253.9 | 7555 | 24.9 | 166.6 | 585 | 4.7 | 916.2 | 6814 | 19.8 | 688.8 | 1873 | 9.2 |
| 25 | [28] | 1061.7 | 6695 | 18.0 | 239.9 | 366 | 2.5 | 693.0 | 6023 | 14.6 | 540.8 | 1674 | 6.9 |
| 26 | [28] | 1140.1 | 6019 | 15.0 | 291.1 | 267 | 2.0 | 1156.4 | 5523 | 11.5 | 646.7 | 522 | 1.8 |
| 27 | [29] | 1465.6 | 8366 | 31.6 | 89.2 | 334 | 3.3 | 1613.4 | 8370 | 31.0 | 406.2 | 607 | 4.6 |
| 28 | [29] | 1340.4 | 7621 | 24.6 | 240.3 | 264 | 2.6 | 1228.6 | 7548 | 24.8 | 608.9 | 891 | 6.0 |
| 29 | [30] | 1206.5 | 7408 | 22.5 | 209.9 | 294 | 2.5 | 849.1 | 7102 | 21.6 | 480.0 | 1199 | 7.0 |
| 30 | [31] | 1221.3 | 7414 | 22.9 | 272.1 | 372 | 3.2 | 1042.3 | 7127 | 21.2 | 660.2 | 1107 | 5.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bondarchuk, S. A Facile Method for Assessing the Change in Detonation Properties during Chemical Functionalization: The Case of NH2→NHNO2 and NH2→=N+=N− Conversions. Chem. Proc. 2022, 12, 48. https://doi.org/10.3390/ecsoc-26-13566
Bondarchuk S. A Facile Method for Assessing the Change in Detonation Properties during Chemical Functionalization: The Case of NH2→NHNO2 and NH2→=N+=N− Conversions. Chemistry Proceedings. 2022; 12(1):48. https://doi.org/10.3390/ecsoc-26-13566
Chicago/Turabian StyleBondarchuk, Sergey. 2022. "A Facile Method for Assessing the Change in Detonation Properties during Chemical Functionalization: The Case of NH2→NHNO2 and NH2→=N+=N− Conversions" Chemistry Proceedings 12, no. 1: 48. https://doi.org/10.3390/ecsoc-26-13566
APA StyleBondarchuk, S. (2022). A Facile Method for Assessing the Change in Detonation Properties during Chemical Functionalization: The Case of NH2→NHNO2 and NH2→=N+=N− Conversions. Chemistry Proceedings, 12(1), 48. https://doi.org/10.3390/ecsoc-26-13566





