Abstract
Schiff bases have been important compounds ever since their discovery and are both found in nature and synthesized in the laboratory. They participate in a variety of synthetic processes and possess desirable biological activity, including antibacterial, anti-inflammatory, antioxidant, and anticancer activity, among others. In this study, eight Schiff bases derived from the reaction of 4-aminoantipyrine with various cinnamaldehydes have been synthesized and characterized. All derivatives were tested in vitro on several human carcinoma cell lines to determine their antitumor activity and against different bacteria strains of clinical and food industry importance to evaluate their antibacterial activity. Several of the Schiff bases evaluated inhibited tumor cell growth in a dose-dependent manner. The compound that exhibited the most activity against all cell lines had IC50 values of less than 18 μM. On the other hand, during the evaluation of the antibacterial activity, only two Schiff base derivatives showed interesting antibacterial effects, with MIC values under 250 μM. These two Schiff base derivatives mainly exhibited a bacteriostatic effect against most of the studied bacterial strains. It is interesting to note that the same Schiff base presents the best activity in both biological evaluations.
1. Introduction
Schiff bases, also known as imines or azomethines, have gained a lot of interest due to their wide range of applications, including pigments and dyes, catalysts, polymer stabilizers, luminescence chemosensors, corrosion inhibitors [1], organic synthesis intermediates, and new drug development [2,3]. The electron-donating nitrogen in the azomethine bond also makes these compounds L-type ligands that can interact with virtually any metal to create complexes [4,5]. One of the factors contributing to the popularity of Schiff bases in organic chemistry may be the simplicity of their synthesis. Condensation of primary amines with carbonyl compounds under reflux conditions can yield a large number of compounds in high yields; however, new methodologies have been developed that include the use of microwave, solvent-free synthesis or the use of Lewis or Bronsted–Lowry acids as catalysts, such as ZnCl2, TiCl4, alumina, P2O5/Al2O3, or Er(OTf)3 [6].
Schiff bases have shown a wide range of biological activities [7] (Scheme 1), such as antileishmanial [8], analgesic [9], anti-inflammatory [10], antioxidant [11], antiviral [12], antifungal [13], and antibacterial activities [3], and for their biological activities, the imine or azomethine group (>C=N-) seems to be crucial.
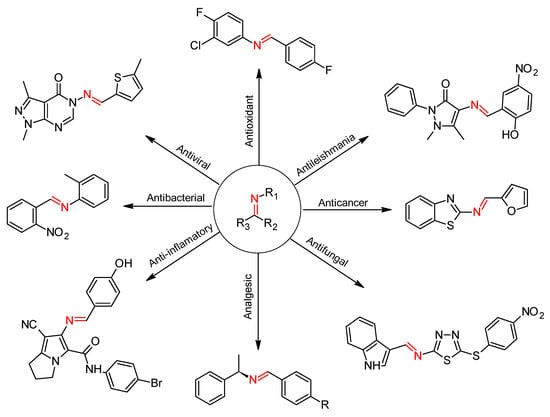
Scheme 1.
Different bioactive Schiff base derivatives.
Infections caused by the development of antimicrobial resistance (AMR) to existing antibiotics are a serious public health problem all over the world. According to recent data, an estimated 4.95 million people died from diseases associated with AMR in 2019 [14,15]. Moreover, the fast spread of multi-resistant bacteria worldwide is a serious topic that needs an immediate response [16]. Clinical strains and those associated with foodborne diseases become more dangerous due to the widespread and uncontrolled use of antibiotics for human health and livestock [17,18,19]. As a result, the necessity for effective treatments has been a driving factor in the study, design, and synthesis of novel biologically active compounds. Schiff bases have been reported to offer better anti-tumoral properties against a broad variety of tumor cells compared to standard chemotherapeutic drugs, such as cisplatin and doxorubicin [20]. They are capable of interacting with the nuclear DNA and trigger apoptosis, as well as modulating the intracellular redox equilibrium without significantly interfering with normal cell growth. Such mechanisms are particularly relevant in the context of cancer, where drug resistance and the high toxicity of conventional treatments has encouraged scientists to develop new and more effective anti-tumoral drugs [21].
Among Schiff bases, those derived from 4-aminoantipyrine have been shown to have interesting bioactivities, and the synthesis of new derivatives has caught the interest of many researchers, particularly in medicinal chemistry, due to their broad-spectrum biological activities [22,23]. Based on the facts presented above, this study was conducted in order to identify new antibacterial and anticancer drug candidate compounds. The synthesis of a variety of Schiff base derivatives from 4-aminoantipyrine with different cinnamaldehydes is described. To determine the biological significance of the synthesized compounds, we tested them against several bacteria strains of clinical and food industry interest, as well as against several human carcinoma cell lines.
2. Methods
2.1. General
All solvents and reagents were acquired from Sigma-Aldrich (St. Louis, MO, USA) and were used without further purification. All melting points are uncorrected and were determined on a Fisher-Johns analog melting point apparatus. FTIR spectra were recorded by a Perkin Elmer FTIR Spectrum One using an ATR system (4000–650 cm−1). The 1H and 13C NMR spectra were recorded at 298 K on a Bruker Advance 500 MHz spectrometer equipped with a z-gradient, triple-resonance (1H, 13C, 15N) cryoprobe using DMSO-d6 or CDCl3 as solvents. Chemical shifts are expressed in ppm with TMS as an internal reference (TMS, δ = 0 ppm) for protons. Reactions were monitored by TLC on silica gel using ethyl acetate/hexane mixtures as a solvent and compounds visualized by UV lamp. The reported yields are for the purified material and are not optimized.
2.2. Synthesis
All Schiff bases 3 were synthesized according to the reported procedures by our research group (Scheme 2) [8]. The synthesis of the Schiff base derivatives 3a–h starts with a mixed equimolar reaction of 4-amino-1,5-dimethyl-2-phenylpyrazol-3-one (1) (1.722 mmol) and (1.722 mmol) of substituted cinnamaldehydes 2a–h, dissolved in 5.00 mL of EtOH, and the mixture was refluxed for 1h to 24h. The exception was the reaction of 3f, where reflux was not used. The progress of the reaction was monitored by TLC. The precipitates formed were collected by filtration and purified by recrystallization with ethanol, then the products were dried under vacuum to obtain the pure compounds.
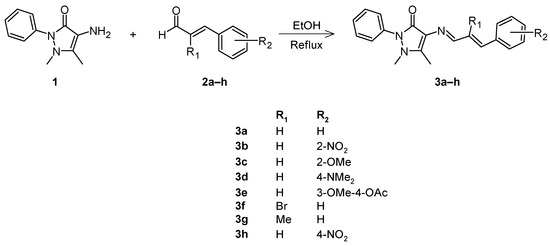
Scheme 2.
General reaction for the synthesis of Schiff bases 3a–h.
4-[(3-Phenyl)allylideneamino]-1,5-dimethyl-2-phenyl-1H-pyrazol-3(2H)-one (3a); yield 90% as yellow crystals; m.p. 162–163 °C (Lit [8] 165.5–165.9 °C); 1H-NMR (300 MHz, DMSO-d6) δ 9.40 (d, J = 8.2 Hz, 1H), 7.64 (dd, J = 8.3, 1.4 Hz, 2H), 7.57–7.49 (m, 2H), 7.43–7.29 (m, 6H), 7.11 (d, J = 16.1 Hz, 1H), 7.01 (dd, J = 16.1, 8.3 Hz, 1H), 3.17 (s, 3H), 2.39 (s, 3H).
4-[3-(2-Nitrophenyl)allylideneamino]-1,5-dimethyl-2-phenyl-1H-pyrazol-3(2H)-one (3b); yield 84.8% as red crystals; m.p. 164–165 °C; 1H NMR (500 MHz, CDCl3) δ 9.56 (d, J = 9.0 Hz, 1H), 7.92 (dd, J = 8.2, 1.0 Hz, 1H), 7.72 (d, J = 7.9 Hz, 1H), 7.57 (t, J = 7.4 Hz, 1H), 7.50 (d, J = 15.8 Hz, 1H), 7.45 (t, J = 7.9 Hz, 2H), 7.40 (dt, J = 7.7, 1.1 Hz, 1H), 7.37 (dd, J = 8.0, 1.0 Hz, 2H), 7.30 (t, J = 7.4 Hz, 1H), 6.94 (dd, J = 15.8, 9.0 Hz, 1H), 3.14 (s, 3H), 2.41 (s, 3H); 13C NMR (126 MHz, CDCl3) δ 160.4, 158.3, 151.8, 148.0, 135.3, 134.7, 134.5, 133.1, 132.0, 129.3, 128.9, 128.3, 127.2, 124.9, 124.7, 118.9, 35.7, 10.1; FTIR (cm−1) 3052, 1642, 1556, 1339, 977.
4-[3-(2-Methoxyphenyl)allylideneamino]-1,5-dimethyl-2-phenyl-1H-pyrazol-3(2H)-one (3c); yield 85.7% as yellow crystals; m.p. 175–176 °C; 1H NMR (500 MHz, CDCl3) δ 9.57 (d, J = 9.2 Hz, 1H), 7.55 (dd, J = 7.7, 1.6 Hz, 1H), 7.45 (dd, J = 8.3, 7.4 Hz, 2H), 7.43 (d, J = 16.1 Hz, 1H), 7.39 (dd, J = 8.6, 1.3 Hz, 2H), 7.29 (tt, J = 7.1, 1.3 Hz, 1H), 7.25 (ddd, J = 8.4, 7.5, 1.7 Hz, 1H), 7.03 (dd, J = 16.1, 9.2 Hz, 1H), 6.94 (td, J = 7.6, 0.9 Hz, 1H), 6.87 (dd, J = 8.3, 0.8 Hz, 1H), 3.84 (s, 3H), 3.09 (s, 3H), 2.40 (s, 3H); 13C NMR (126 MHz, CDCl3) δ 160.9, 160.8, 157.5, 151.3, 136.6, 135.0, 130.6, 130.0, 129.2, 127.4, 126.8, 125.5, 124.3, 120.8, 119.6, 111.2, 55.6, 35.9, 10.2; FTIR (cm−1) 3035, 1642, 1237, 1049, 990, 764.
4-[3-(4-Dimethylaminophenyl)allylideneamino]-1,5-dimethyl-2-phenyl-1H-pyrazol-3(2H)-one (3d); yield 97.5% as orange crystals; m.p. 179–180 °C; 1H NMR (500 MHz, CDCl3) δ 9.52 (d, J = 9.1 Hz, 1H), 7.46 (t, J = 7.8 Hz, 2H), 7.40 (dd, J = 8.5, 1.4 Hz, 2H), 7.39 (d, J = 8.8 Hz, 2H), 7.29 (tt, J = 7.0, 1.3 Hz, 1H), 6.98 (d, J = 15.8 Hz, 1H), 6.81 (dd, J = 15.7, 9.1 Hz, 1H), 6.67 (d, J = 8.9 Hz, 2H), 3.09 (s, 3H), 2.99 (s, 6H), 2.41 (s, 3H); 13C NMR (126 MHz, CDCl3) δ 161.09, 161.07, 151.02, 150.96, 142.4, 135.1, 129.2, 128.8, 126.8, 125.8, 124.7, 124.3, 120.0, 112.2, 40.4, 36.2, 10.2; FTIR (cm−1) 3019, 1649, 1600, 1367, 1147, 980, 808.
4-[3-(4-Acetoxy-3-methoxyphenyl)allylideneamino]-1,5-dimethyl-2-phenyl-1H-pyrazol-3(2H)-one (3e); yield 81.7% as yellow crystals; m.p. 240–241 °C; 1H NMR (500 MHz, CDCl3) δ 9.55 (d, J = 8.6 Hz, 1H), 7.47 (dd, J = 8.3, 7.4 Hz, 2H), 7.39 (dd, J = 8.5, 1.2 Hz, 2H), 7.32 (tt, J = 7.0, 1.2 Hz, 1H), 7.11 (d, J = 1.8 Hz, 1H), 7.07 (dd, J = 8.2, 1.8 Hz, 1H), 7.01 (d, J = 8.1 Hz, 1H), 7.00 (d, J = 15.9 Hz, 1H), 6.93 (dd, J = 15.9, 8.6 Hz, 1H), 3.86 (s, 3H), 3.14 (s, 3H), 2.43 (s, 3H), 2.31 (s, 3H); 13C NMR (126 MHz, CDCl3) δ 169.0, 160.8, 159.5, 151.5, 151.4, 140.4, 140.4, 135.6, 134.9, 130.7, 129.3, 127.1, 124.7, 123.2, 120.5, 119.4, 110.6, 56.0, 35.9, 20.8, 10.2. 13C NMR (126 MHz, CDCl3) δ 169.0, 160.8, 159.5, 151.5, 151.4, 140.4, 140.4, 135.6, 134.9, 130.7, 129.3, 127.1, 124.7, 123.2, 120.5, 119.4, 110.6, 56.0, 35.9, 20.8, 10.2; FTIR (cm−1) 3011, 1755, 1640, 1417, 1289, 1199, 1032, 991.
4-[2-bromo-(3-phenyl)allylideneamino]-1,5-dimethyl-2-phenyl-1H-pyrazol-3(2H)-one (3f); yield 86.9% as yellow crystals; m.p. 149–150 °C; 1H NMR (500 MHz, CDCl3) δ 9.48 (s, 1H), 7.86 (d, J = 7.5 Hz, 2H), 7.50–7.43 (m, 3H), 7.41–7.36 (m, 4H), 7.36–7.33 (m, 2H), 7.34–7.28 (m, 2H), 3.12 (s, 3H), 2.46 (s, 3H); 13C NMR (126 MHz, CDCl3) δ 160.3, 155.0, 152.3, 139.0, 135.1, 134.6, 130.0, 129.2, 129.1, 128.3, 127.1, 125.7, 124.6, 117.9, 35.5, 10.1; FTIR (cm−1), 3066, 1644, 1591, 1492, 1310, 1136, 756, 693.
4-[2-Methyl-(3-phenyl)allylideneamino]-1,5-dimethyl-2-phenyl-1H-pyrazol-3(2H)-one (3g); yield 94.59% as yellow crystals; m.p. 169–170 °C; 1H NMR (500 MHz, CDCl3) δ 9.51 (s, 1H), 7.47 (dd, J = 8.3, 7.3 Hz, 2H), 7.45 (d, J = 7.2 Hz, 2H), 7.41 (dd, J = 8.5, 1.3 Hz, 2H), 7.38 (t, J = 7.7 Hz, 2H), 7.30 (tt, J = 7.4, 1.3 Hz, 1H), 7.27 (tt, J = 7.3, 1.3 Hz, 1H), 6.94 (s, 1H), 3.10 (s, 3H), 2.43 (s, 3H), 2.24 (d, J = 1.2 Hz, 3H); 13C NMR (126 MHz, CDCl3) δ 162.6, 161.0, 151.9, 139.2, 138.5, 137.3, 135.1, 129.6, 129.2, 128.4, 127.5, 126.8, 124.3, 119.3, 36.1, 12.3, 10.1; FTIR (cm−1) 3066, 1640, 1587, 1480, 1455, 1302, 754, 693.
4-[3-(4-Nitrophenyl)allylideneamino]-1,5-dimethyl-2-phenyl-1H-pyrazol-3(2H)-one (3h); yield 98.2% as red crystals; m.p. 217–218 °C; 1H NMR (500 MHz, CDCl3) δ 9.55 (d, J = 8.5 Hz, 1H), 8.18 (d, J = 8.8 Hz, 2H), 7.60 (d, J = 8.8 Hz, 2H), 7.47 (dd, J = 8.3, 7.4 Hz, 2H), 7.37 (dd, J = 8.5, 1.1 Hz, 2H), 7.33 (tt, J = 7.0, 1.1 Hz, 1H), 7.08 (dd, J = 16.0, 8.5 Hz, 1H), 7.01 (d, J = 16.0 Hz, 1H), 3.17 (s, 3H), 2.43 (s, 3H); 13C NMR (126 MHz, CDCl3) δ 160.3, 157.7, 151.7, 147.3, 142.9, 137.3, 134.7, 134.5, 129.3, 127.6, 127.3, 124.8, 124.1, 118.8, 35.5, 10.0; FTIR (cm−1) 3071, 1645, 1511, 1335, 972, 825.
2.3. Biological Evaluation
2.3.1. Evaluation of Antitumoral Activity
HeLa (human cervical carcinoma), HCT116 and HT29 (human colorectal carcinoma), SK-MEL103 (human melanoma), MDA-MB-231 (human breast carcinoma), and NIH3T3 (mouse NIH/Swiss embryo fibroblasts) were obtained from ATCC and cultured in Dulbecco’s modified eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) (Eurobio, Les Ulis, France) and 1% penicillin/streptomycin (Thermo Fisher Scientific, Gibco, Miami, FL, USA). All cell lines were maintained at 37 °C in a humidified atmosphere at 5% CO2.
To assay the effect of the compounds on cell proliferation, cells were seeded at a density of 1 × 104 cells/well in 96-well plates and incubated for 72 h with 100 μL of the eight Schiff bases at 4–250 μM final concentrations. Derivatives were dissolved in DMSO at a stock concentration of 20 mM. The final working concentration of DMSO (<1%, v/v) did not affect cell growth. After the incubation period, the MTT (thiazolyl blue tetrazolium bromide) dye assay (Sigma, St. Louis, MO, USA) was used following the standard protocol provided by the supplier. Briefly, 10 μL of MTT solution (5 mg/mL) was added to each well. After 1–2 h incubation in a humidified atmosphere, media was removed and 50 μL of DMSO were added to each well to solubilize the formazan crystals. Agitation was performed for 5 min before measuring the absorbance with a Cytation5 multi-mode detection system (BioTek, Winooski, VT, USA) at 570 nm. Each data point was generated from triplicate samples, and experiments were repeated four times. To determine the concentration of compound inhibiting 50% of cell proliferation (IC50), dose-response curves were generated in GraphPad Prism (GraphPad Software, San Diego, CA, USA) using untreated cells as 100% cell proliferation control.
2.3.2. Evaluation of Antibacterial Activity
The antibacterial activity of all synthesized Schiff bases was tested against the Gram-positive bacteria Staphylococcus aureus ATCC 25923, Enterococcus faecalis ATCC 29212, Bacillus cereus, Listeria monocytogenes ATCC 13932 and the Gram-negative bacteria Escherichia coli ATCC 25922, using the microdilution method [24].
The bacterial inoculum was prepared in brain–heart infusion broth (BHI) to a final cell density of 5 × 105 cfu/mL. Stock solutions of the tested compounds were prepared by dissolving them in DMSO at 10 mM. Tested volumes were adjusted so the final concentration of the DMSO in each well was always 2.5% v/v. This concentration was shown to not affect bacterial growth previously [8]. As control, bacterial cells were grown with 2.5% DMSO to rule out any potential growth inhibitory effect. Additionally, several antibiotics were used as controls for growth inhibition at the recommended working concentrations for the tested strains (Table 1). Both BHI alone and supplemented with the compounds at different concentrations were used as blanks.

Table 1.
List of antibiotics and concentrations used as controls during the evaluation of antibacterial activity.
The range of concentrations (0.5 µM–250 µM) used for the Schiff bases was selected based on previous findings for Schiff base derivatives [8]. Drug sensitivities were assessed via the microdilution method [25] and according to the Clinical and Laboratory Standards Institute (CSLI) guidelines [24], with the following modifications: first, the compounds were serially diluted in DMSO, then 5 µL of each dilution was added to 195 µL of bacterial suspension (5 × 105 cfu/mL) to a total volume of 200 µL. The plates were then incubated at 37 °C for 20 h with constant shaking at 300 cpm (double orbital setting), and the OD600 was monitored every 30 min in a Cytation5 multi-mode detection system (BioTek). The minimal inhibitory concentration (MIC) was determined after tracking the bacterial growth over 20 h in samples exposed to the tested compound at different concentrations. The MIC was defined as the lowest concentration of the antibacterial agent, which completely inhibited the growth of the microorganism as determined by the optical density at 600 nm. These assays were performed at least in triplicate.
To determine if the inhibitory effect was either bactericidal or bacteriostatic, the bacteria were grown with the respective compound at the established MIC, and in a compound-free medium for 20 h in a microplate with the same shaking and temperature conditions as above. Finally, the bacterial suspension was pelleted, washed in 500 µL of BHI, and plated as a drop on plain BHI agar overnight at 37 °C. Bacterial survival was registered as bacteriostatic effect, whereas bacterial absence was registered as bactericidal effect.
3. Results and Discussion
3.1. Synthesis of Schiff Base of 4-Aminoantipyrine
Schiff bases 3 were synthesized as previously reported [8]. The condensation of 4-amino-1,5-dimethyl-2-phenylpyrazol-3-one (1) with various cinnamaldehydes 2a–h, using ethanol as solvent, affords the corresponding Schiff base 3a–h in good to excellent yield as pure products after recrystallization in EtOH (Scheme 2) (Table 2). All compounds were characterized, and all the data obtained agreed with the proposed structures. The 1H-NMR spectra for 3a–h shows a doublet between 9.40 and 9.57 ppm, corresponding to the azomethine –CH=N proton, except for 3f and 3g, which appear as a singlet at 9.48 and 9.51 ppm, respectively. Despite the type of the substituent, the signal shifts downfield when the substituent is in position 2 compared to when it is in another position.

Table 2.
General reaction for the synthesis of Schiff bases 3a–h.
3.2. Antitumor Activity Evaluation
Cells were exposed to different concentrations of each derivative for 72 h and proliferation was monitored through the MTT assay. Based on the IC50 values (Table 3) obtained in tumor cells compared to the IC50 values obtained in non-tumor cells, the most efficient derivatives were 3h > 3c. The compound with the highest toxicity profile was 3f; in contrast, derivatives 3e and 3g did not show any effect against tumor or non-tumor cells.

Table 3.
Inhibitory concentration values (IC50) a of Schiff bases 3a–h against tumor and non-tumor cell lines at 72 h.
3.3. Antibacterial Activity Evaluation
Antibacterial activities were determined by testing the eight Schiff bases against the Gram-positive bacteria Staphylococcus aureus, Enterococcus faecalis, Bacillus cereus, and Listeria monocytogenes and the Gram-negative bacteria Escherichia coli. However, only the Schiff bases 3f and 3h showed inhibition of the bacterial growth, and their range of minimum inhibitory concentration (MIC) are detailed in Table 4. MIC values above 250 µM were not considered as effective and were labeled as “non-effective” (NE).

Table 4.
Minimal inhibitory concentration (MIC) a and type of inhibition determined by growth kinetics over 20 h (OD600) after serial microdilution in 96-well plates for Schiff bases.
Compounds 3a, 3b, 3c, 3d, 3e, and 3g did not show any activity against the tested bacteria, whereas 3f showed antibacterial activity for all the tested strains (<100 µM). The lowest MIC value identified so far (15.6 µM) corresponds to 3f against E. coli. In general, 3f showed increased antibacterial activity compared to 3h within the same strain, with MIC values at least 2.5 times lower. This result could be attributed to the presence of a bromine atom in the structure, which is known to have antibacterial properties due to its oxidant potential [26]. On the other hand, when the nitro group is present (3h), the activity is limited to L. monocytogenes and B. cereus, which suggests a different mode of action compared to 3f.
Additionally, after 20 h exposure of the bacteria to the compounds, they were plated on drug-free agar to determine the type of inhibitory effect. All the strains subjected to 3f and 3h showed a bacteriostatic effect, except from L. monocytogenes exposed to compound 3f, which showed a bactericidal effect. All effects are reported in Table 4.
4. Conclusions
The Schiff base derivatives 3a–h can be readily synthesized with high yields by the condensation reaction between 4-aminoantipyrine (1) and various cinnamaldehydes. Schiff base derivatives 3h and 3c inhibited tumor cell proliferation while having no effect on non-tumoral cells utilized as controls. As a result, these compounds show potential as antitumor agents and may benefit from additional research. Furthermore, Schiff bases 3f and, to a lesser extent, 3h have promising activity against different Gram-positive and Gram-negative bacteria and should be investigated further. The antibacterial potential of 3f could be attributed to the oxidative properties of the bromine atom, which benefits pathogen growth inhibition.
Author Contributions
Conceptualization, J.H.-M.; formal analysis, E.A.-L., S.E.C.-P., R.G.-P., J.C.R.-B. and J.H.-M.; investigation, E.A.-L., S.E.C.-P., R.G.-P., J.Z.-M., C.R.-P., J.C.R.-B. and J.H.-M.; writing—original draft preparation, E.A.-L., R.G.-P. and J.Z.-M.; writing—review and editing, S.E.C.-P., R.G.-P. and J.H.-M.; supervision, J.H.-M.; project administration, J.H.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
This study was funded by Universidad UTE and Universidad Técnica Particular de Loja (UTPL).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Okey, N.C.; Obasi, N.L.; Ejikeme, P.M.; Ndinteh, D.T.; Ramasami, P.; Sherif, E.-S.M.; Akpan, E.D.; Ebenso, E.E. Evaluation of some amino benzoic acid and 4-aminoantipyrine derived Schiff bases as corrosion inhibitors for mild steel in acidic medium: Synthesis, experimental and computational studies. J. Mol. Liq. 2020, 315, 113773. [Google Scholar] [CrossRef]
- Raczuk, E.; Dmochowska, B.; Samaszko-Fiertek, J.; Madaj, J. Different Schiff Bases—Structure, Importance and Classification. Molecules 2022, 27, 787. [Google Scholar] [CrossRef] [PubMed]
- Ceramella, J.; Iacopetta, D.; Catalano, A.; Cirillo, F.; Lappano, R.; Sinicropi, M.S. A Review on the Antimicrobial Activity of Schiff Bases: Data Collection and Recent Studies. Antibiotics 2022, 11, 191. [Google Scholar] [CrossRef] [PubMed]
- Catalano, A.; Sinicropi, M.S.; Iacopetta, D.; Ceramella, J.; Mariconda, A.; Rosano, C.; Scali, E.; Saturnino, C.; Longo, P. A Review on the Advancements in the Field of Metal Complexes with Schiff Bases as Antiproliferative Agents. Appl. Sci. 2021, 11, 6027. [Google Scholar] [CrossRef]
- Matela, G. Schiff Bases and Complexes: A Review on Anti-Cancer Activity. Anticancer Agents Med. Chem. 2020, 20, 1908–1917. [Google Scholar] [CrossRef]
- Murtaza, G.; Mumtaz, A.; Khan, F.A.; Ahmad, S.; Azhar, S.; Najam-Ul-Haq, M.; Atif, M.; Khan, S.A.; Maalik, A.; Alam, F.; et al. Recent pharmacological advancements in Schiff bases: A Review. Acta Pol. Pharm.-Drug Res. 2014, 71, 531–535. [Google Scholar]
- Kajal, A.; Bala, S.; Kamboj, S.; Sharma, N.; Saini, V. Schiff Bases: A Versatile Pharmacophore. J. Catal. 2013, 2013, 893512. [Google Scholar] [CrossRef]
- Teran, R.; Guevara, R.; Mora, J.; Dobronski, L.; Barreiro-Costa, O.; Beske, T.; Pérez-Barrera, J.; Araya-Maturana, R.; Rojas-Silva, P.; Poveda, A.; et al. Characterization of Antimicrobial, Antioxidant, and Leishmanicidal Activities of Schiff Base Derivatives of 4-Aminoantipyrine. Molecules 2019, 24, 2696. [Google Scholar] [CrossRef]
- Afridi, H.H.; Shoaib, M.; Al-Joufi, F.A.; Shah, S.W.A.; Hussain, H.; Ullah, A.; Zahoor, M.; Mughal, E.U. Synthesis and Investigation of the Analgesic Potential of Enantiomerically Pure Schiff Bases: A Mechanistic Approach. Molecules 2022, 27, 5206. [Google Scholar] [CrossRef]
- Shawky, A.M.; Abourehab, M.A.S.; Abdalla, A.N.; Gouda, A.M. Optimization of pyrrolizine-based Schiff bases with 4-thiazolidinone motif: Design, synthesis and investigation of cytotoxicity and anti-inflammatory potency. Eur. J. Med. Chem. 2020, 185, 111780. [Google Scholar] [CrossRef]
- Mermer, A.; Demirbas, N.; Uslu, H.; Demirbas, A.; Ceylan, S.; Sirin, Y. Synthesis of novel Schiff bases using green chemistry techniques; antimicrobial, antioxidant, antiurease activity screening and molecular docking studies. J. Mol. Struct. 2019, 1181, 412–422. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Xu, F.-Z.; Zhu, Y.-Y.; Song, B.; Luo, D.; Yu, G.; Chen, S.; Xue, W.; Wu, J. Pyrazolo [3,4-d]pyrimidine derivatives containing a Schiff base moiety as potential antiviral agents. Bioorg. Med. Chem. Lett. 2018, 28, 2979–2984. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Fan, L.; Pan, Z.; Fan, S.; Shi, L.; Li, X.; Zhao, J.; Wu, L.; Yang, G.; Xu, C. Synthesis of Novel Indole Schiff Base Compounds and Their Antifungal Activities. Molecules 2022, 27, 6858. [Google Scholar] [CrossRef] [PubMed]
- Laxminarayan, R. The overlooked pandemic of antimicrobial resistance. Lancet 2022, 399, 606–607. [Google Scholar] [CrossRef] [PubMed]
- Nadimpalli, M.L.; Chan, C.W.; Doron, S. Antibiotic resistance: A call to action to prevent the next epidemic of inequality. Nat. Med. 2021, 27, 187–188. [Google Scholar] [CrossRef]
- World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report: Early Implementation 2020; World Health Organization: Geneva, Switzerland, 2020; p. 180. [Google Scholar]
- Cohen, T.L. The Next Pandemic: A pragmatic and ethical discussion about the looming threat of antibiotic resistance. Voices Bioeth. 2022, 8. [Google Scholar] [CrossRef]
- Vaughn, V.M.; Gandhi, T.N.; Petty, L.A.; Patel, P.K.; Prescott, H.C.; Malani, A.N.; Ratz, D.; McLaughlin, E.; Chopra, V.; Flanders, S.A. Empiric Antibacterial Therapy and Community-onset Bacterial Coinfection in Patients Hospitalized with Coronavirus Disease 2019 (COVID-19): A Multi-hospital Cohort Study. Clin. Infect. Dis. 2021, 72, e533–e541. [Google Scholar] [CrossRef]
- Ghimpețeanu, O.M.; Pogurschi, E.N.; Popa, D.C.; Dragomir, N.; Drăgotoiu, T.; Mihai, O.D.; Petcu, C.D. Antibiotic Use in Livestock and Residues in Food—A Public Health Threat: A Review. Foods 2022, 11, 1430. [Google Scholar] [CrossRef]
- Iacopetta, D.; Ceramella, J.; Catalano, A.; Saturnino, C.; Bonomo, M.G.; Franchini, C.; Sinicropi, M.S. Schiff Bases: Interesting Scaffolds with Promising Antitumoral Properties. Appl. Sci. 2021, 11, 1877. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, L.; Xie, N.; Nice, E.C.; Zhang, T.; Cui, Y.; Huang, C. Overcoming cancer therapeutic bottleneck by drug repurposing. Signal Transduct. Target. Ther. 2020, 5, 113. [Google Scholar] [CrossRef]
- Reşit, Ç.; Başaran, E.; Boğa, M.; Erdoğan, Ö.; Çınar, E.; Çevik, Ö. Schiff Base Derivatives of 4-Aminoantipyrine as Promising Molecules: Synthesis, Structural Characterization, and Biological Activities. Russ. J. Bioorg. Chem. 2022, 48, 334–344. [Google Scholar] [CrossRef]
- Rashmi, A.; Rishi, S.; Abhishek, T.; Ajmer Singh, G.; Balraj, S.; Sandeep, A.; Rajwinder, K. Design and synthesis of novel 4-aminophenazone Schiff bases by grinding technique as prospective anti-inflammatory agents. J. Appl. Pharm. Sci. 2021, 11, 48–53. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018; ISBN 1562388363. [Google Scholar]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Gottardi, W.; Klotz, S.; Nagl, M. Superior bactericidal activity of N-bromine compounds compared to their N-chlorine analogues can be reversed under protein load. J. Appl. Microbiol. 2014, 116, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).