Use of Oxidative Coupling Strategy as a Means to Increase In Vitro Antioxidant Activity of Vanillin Derivatives †
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Vanillic Dimers
2.2. Evaluation of Antioxidant Properties
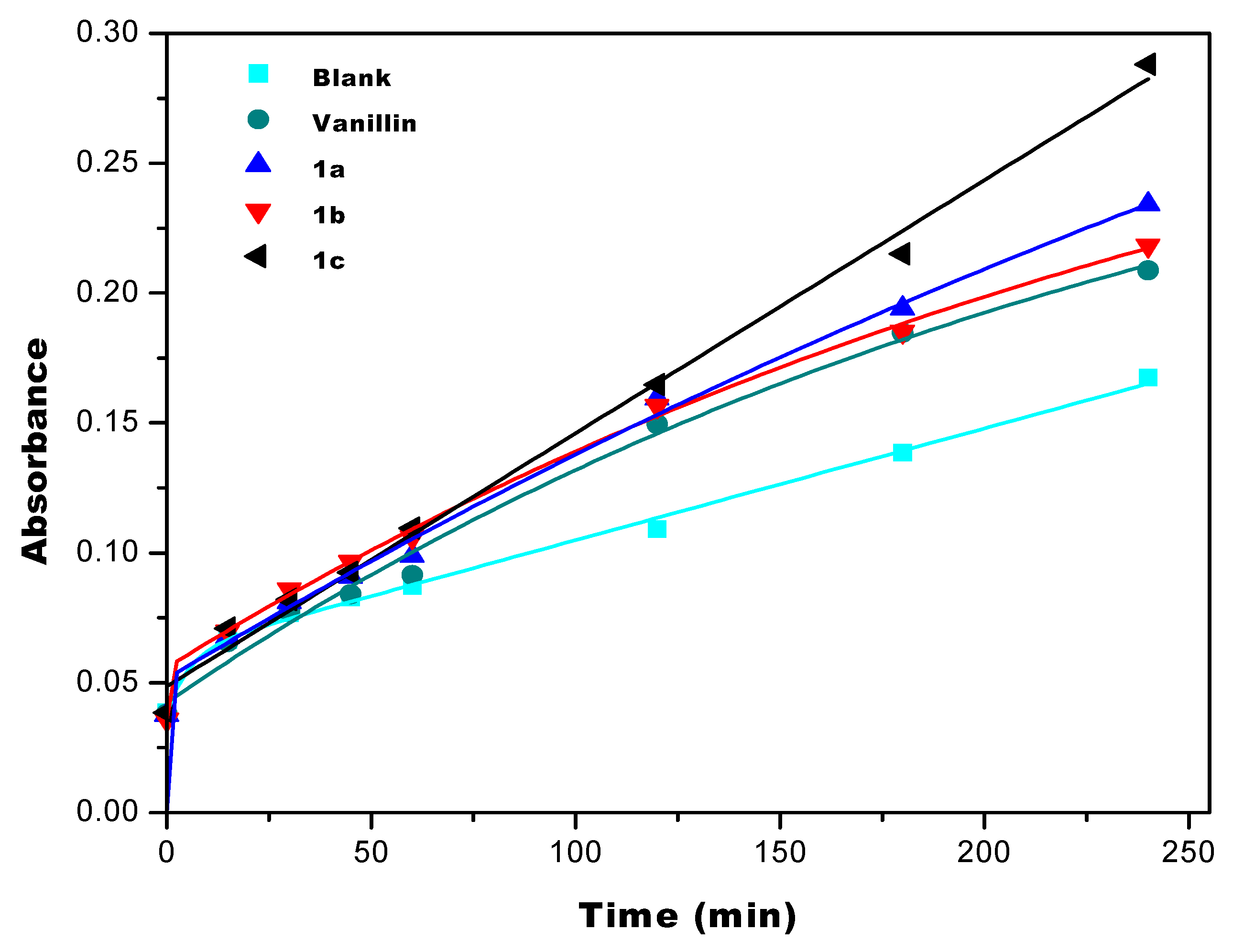
2.2.1. Reducing Power Method (RP)
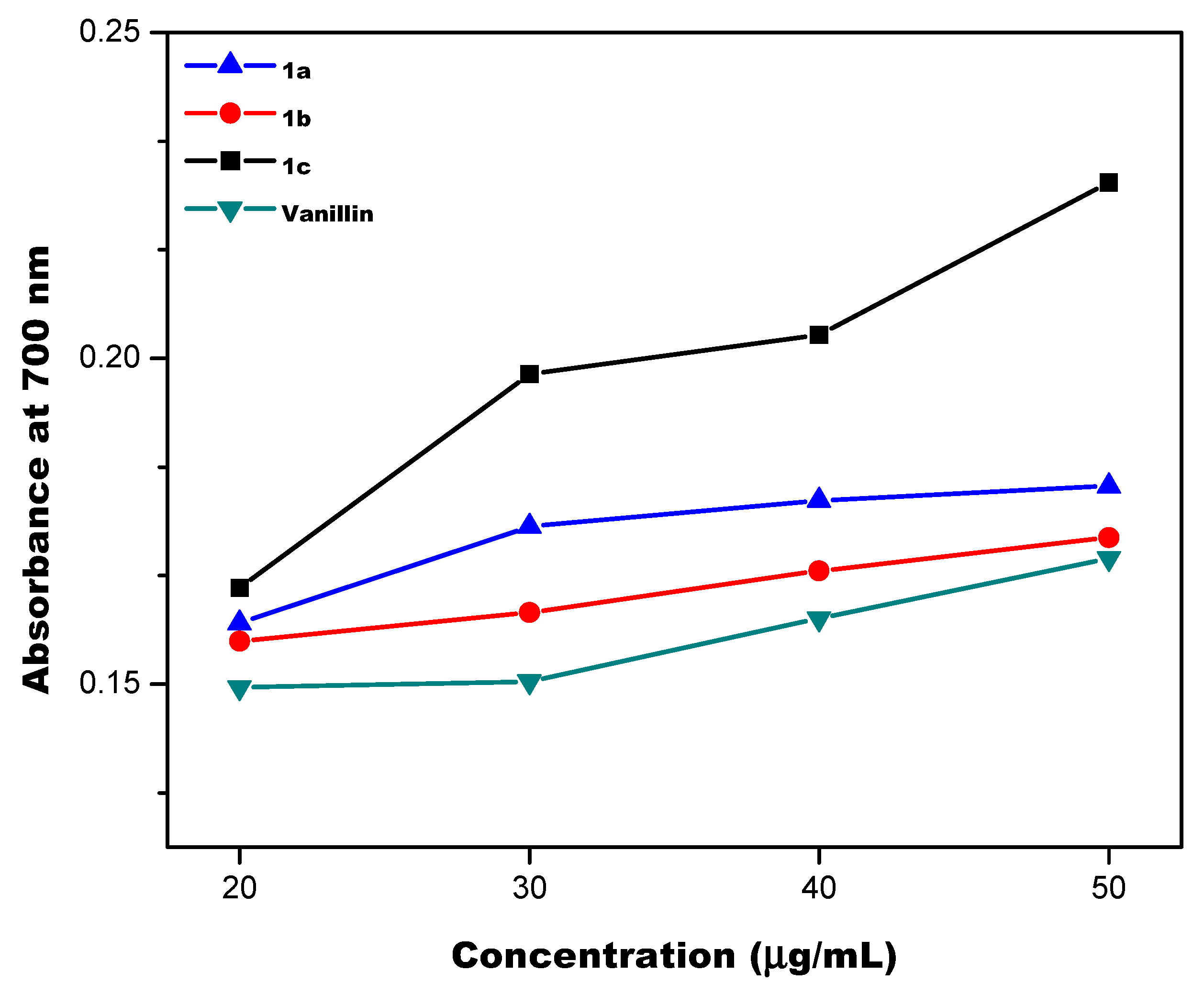
2.2.2. Nitric Oxide Scavenging Activity
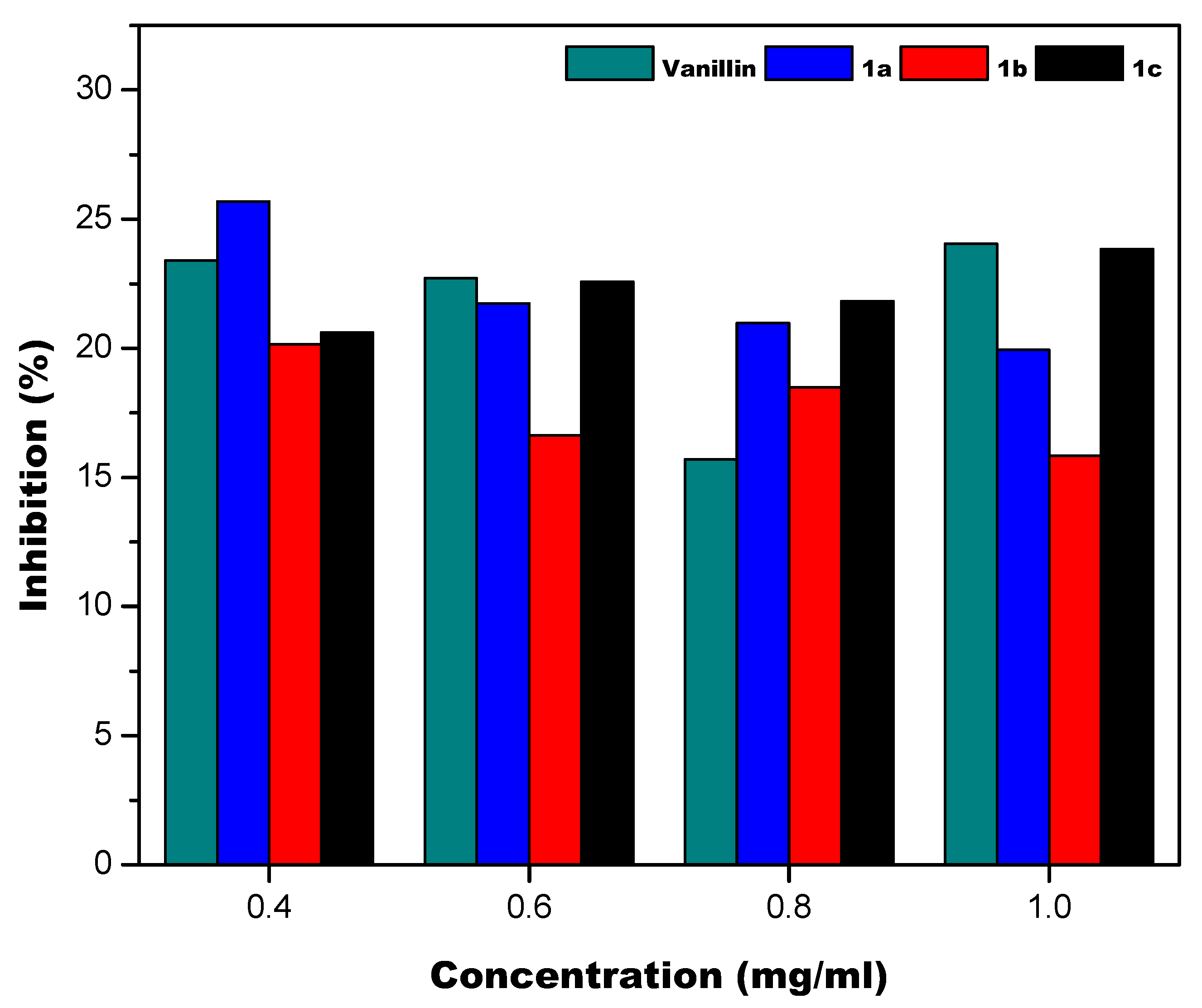
2.2.3. Thiobarbituric Acid Reactive Substances Method (TBARS)
3. Results
3.1. Preparation of Vanillic Dimers
3.2. Antioxidant Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Betteridge, D.J. What is Oxidative Stress? Metabolism 2000, 49, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Jauniaux, E. Oxidative stress. Best. Pract. Res.Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Cutler, R.G. High concentration of antioxidants may not improve defense against oxidative stress. Arch. Gerontol. Geriatr. 1993, 17, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Dubois-Deruy, E.; Peugnet, V.; Turkieh, A.; Pinet, F. Oxidative Stress in Cardiovascular Diseases. Antioxidants 2020, 9, 864. [Google Scholar] [CrossRef] [PubMed]
- Tai, A.; Sawano, T.; Yazama, F.; Ito, H. Evaluation of antioxidant activity of vanillin by using multiple antioxidant assays. Biochim. Biophys. Acta 2011, 1810, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, M.; Burzynska-Pedziwiatr, I.; Wozniak, L.A. A Review of Natural and Synthetic Antioxidants Important for Health and Longevity. Curr. Med. Chem. 2010, 17, 3262–3288. [Google Scholar] [CrossRef] [PubMed]
- Delomenede, M.; Bedos-Belval, F.; Duran, H.; Vindis, C.; Baltas, M.; Nègre-Salvayre, A. Development of Novel Antiatherogenic Biaryls: Design, Synthesis and Reactivity. J. Med. Chem. 2008, 51, 3171–3181. [Google Scholar] [CrossRef] [PubMed]
- De Vasconcelos, D.B.; Lima, A.N.; Philot, E.A.; Scott, A.L.; Ferreira Boza, I.A.; de Souza, A.R.; Morgon, N.H.; Ximenes, V.F. Methyl divanillate: Redox properties and binding affinity with albumin of an antioxidant and potential NADPH oxidase inhibitor. RSC Adv. 2019, 9, 19983–19992. [Google Scholar] [CrossRef] [PubMed]
- Scholtes, J.F.; Trapp, O. Inducing Enantioselectivity in a Dynamic Catalyst by Supramolecular Interlocking. Angew. Chem. Int. Ed. 2019, 58, 6306–6310. [Google Scholar] [CrossRef] [PubMed]
- Oyaizu, M. Studies on products of browning reactions: Antioxidant activities of products of browning reaction prepared from glucosamine. J. Nutrit. 1986, 44, 307–315. [Google Scholar]
- Marcocci, I.; Marguire, J.J.; Droy-lefaiz, M.T.; Packer, L. The nitric oxide scavenging properties of Gingko bioba extract. Biochem. Biophys. Res. Commun. 1994, 201, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Ottolenghi, A. Interaction of ascorbic acid and mitochrondria lipids. Arch. Biochem. Biophys. 1959, 79, 355–363. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutierrez, L.G.; Reinick, A.P.; Ormachea, C.M.; Guntero, V.A.; Ferretti, C.A. Use of Oxidative Coupling Strategy as a Means to Increase In Vitro Antioxidant Activity of Vanillin Derivatives. Chem. Proc. 2022, 12, 31. https://doi.org/10.3390/ecsoc-26-13553
Gutierrez LG, Reinick AP, Ormachea CM, Guntero VA, Ferretti CA. Use of Oxidative Coupling Strategy as a Means to Increase In Vitro Antioxidant Activity of Vanillin Derivatives. Chemistry Proceedings. 2022; 12(1):31. https://doi.org/10.3390/ecsoc-26-13553
Chicago/Turabian StyleGutierrez, Leandro G., Ana P. Reinick, Carla M. Ormachea, Vanina A. Guntero, and Cristián A. Ferretti. 2022. "Use of Oxidative Coupling Strategy as a Means to Increase In Vitro Antioxidant Activity of Vanillin Derivatives" Chemistry Proceedings 12, no. 1: 31. https://doi.org/10.3390/ecsoc-26-13553
APA StyleGutierrez, L. G., Reinick, A. P., Ormachea, C. M., Guntero, V. A., & Ferretti, C. A. (2022). Use of Oxidative Coupling Strategy as a Means to Increase In Vitro Antioxidant Activity of Vanillin Derivatives. Chemistry Proceedings, 12(1), 31. https://doi.org/10.3390/ecsoc-26-13553





