Development of New Effective Methods for the Synthesis of Lembehynes A–C Exhibiting Cytotoxic and Neuritogenic Activity †
Abstract
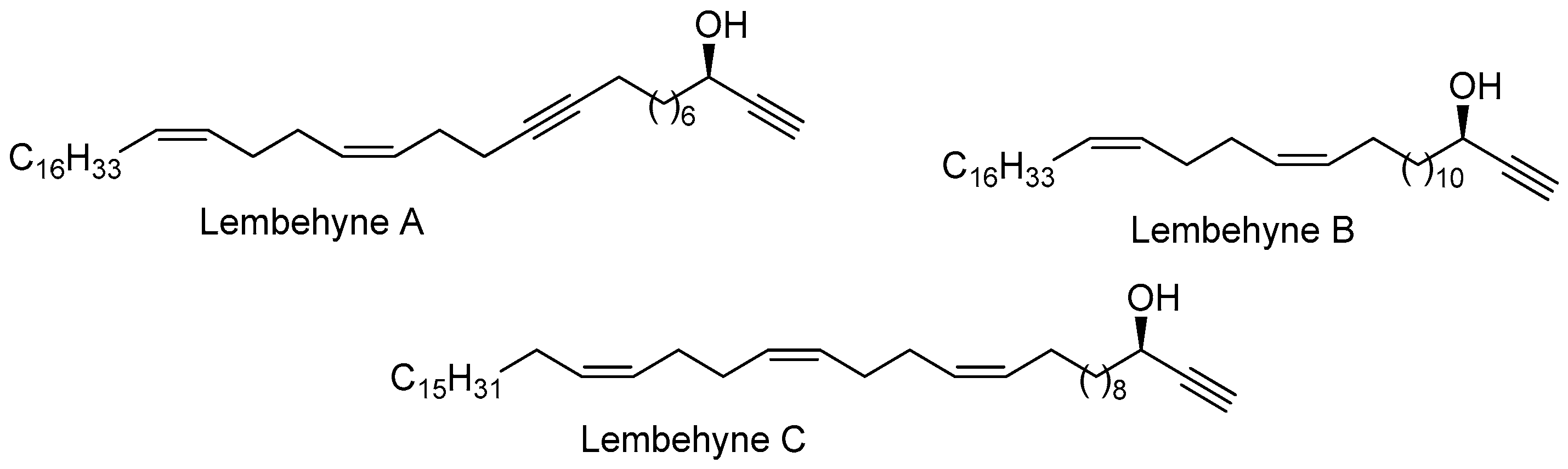
1. Introduction
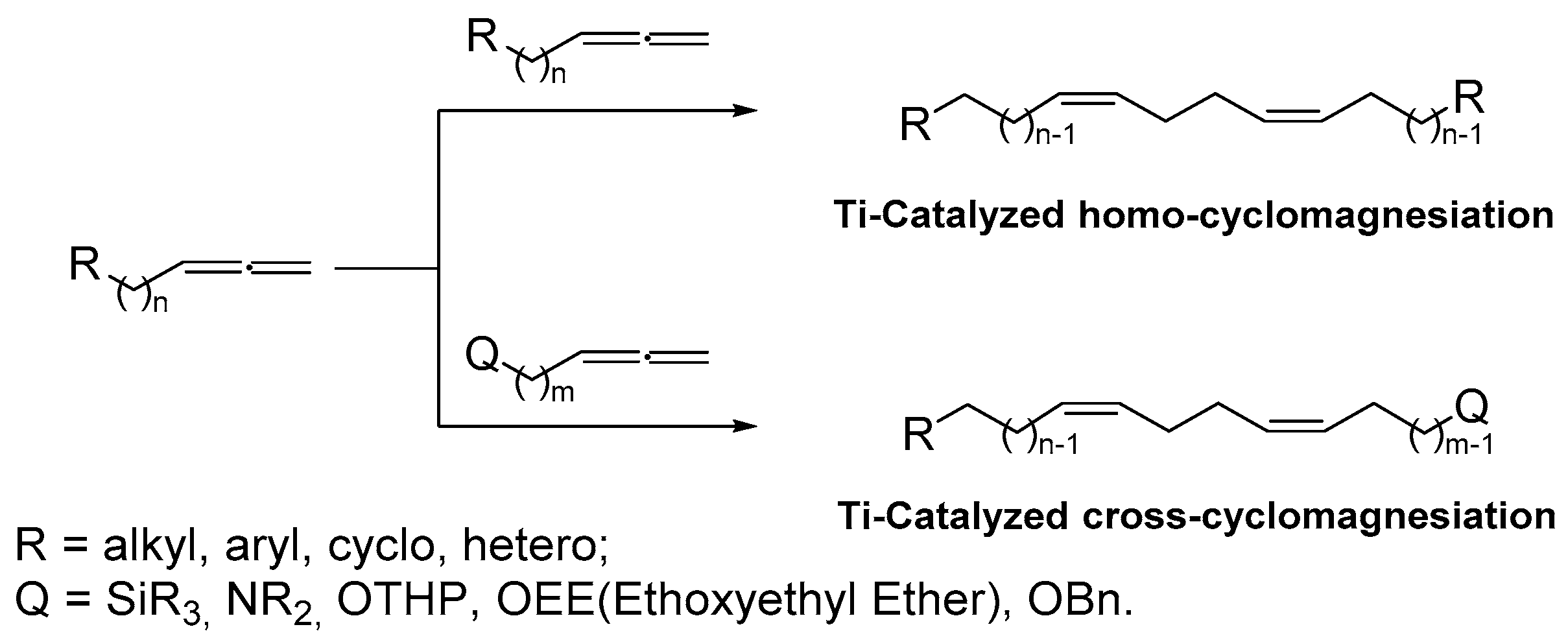
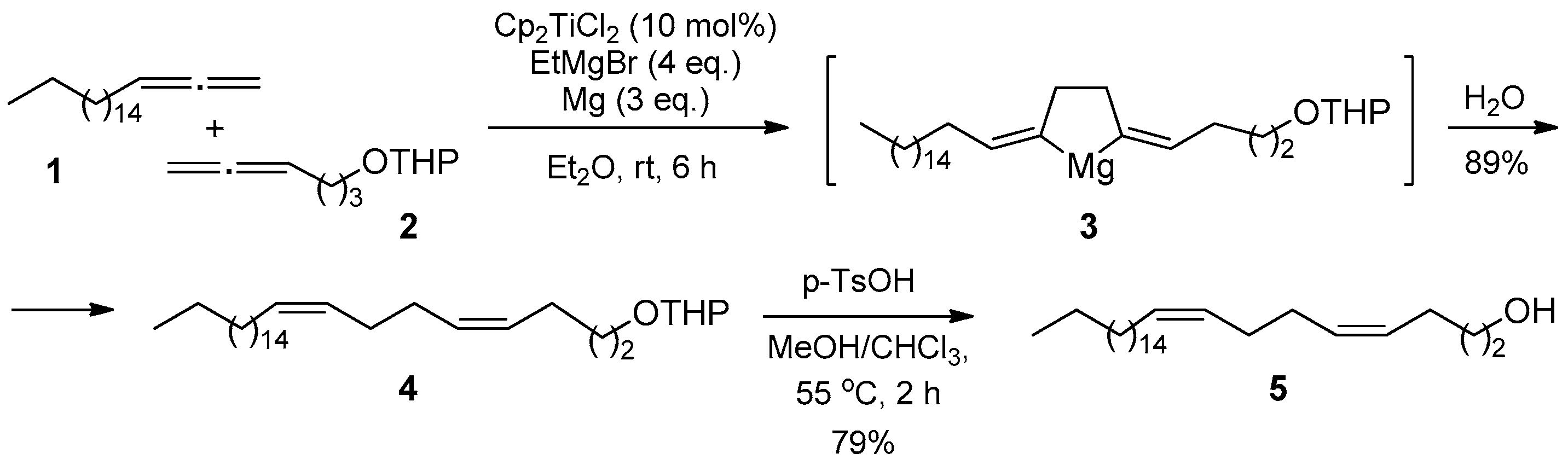
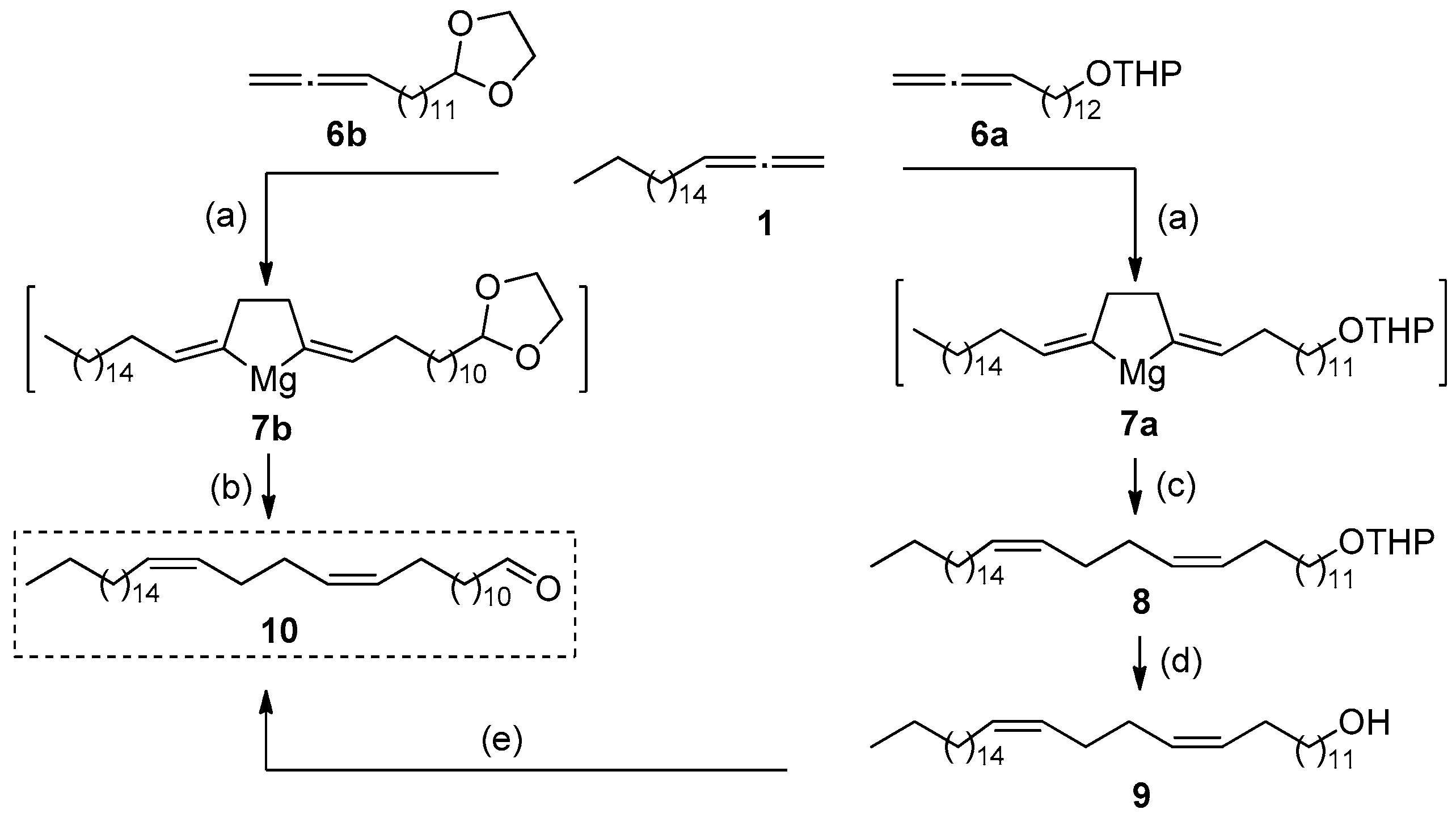
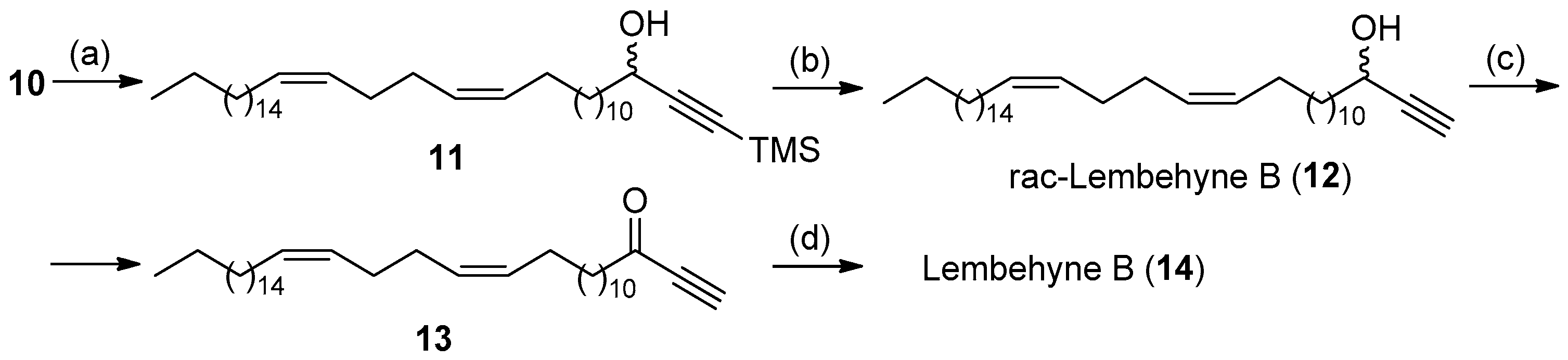
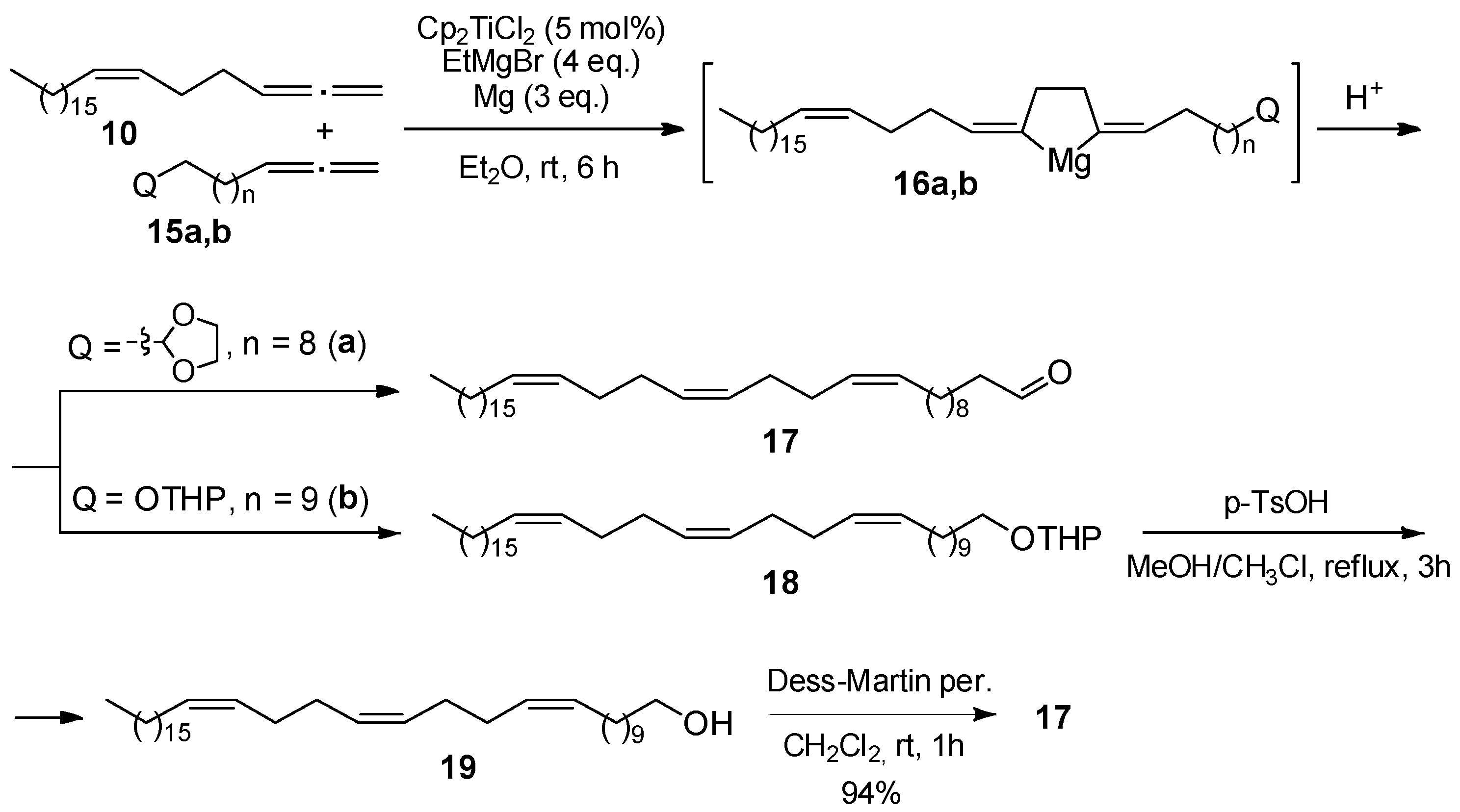
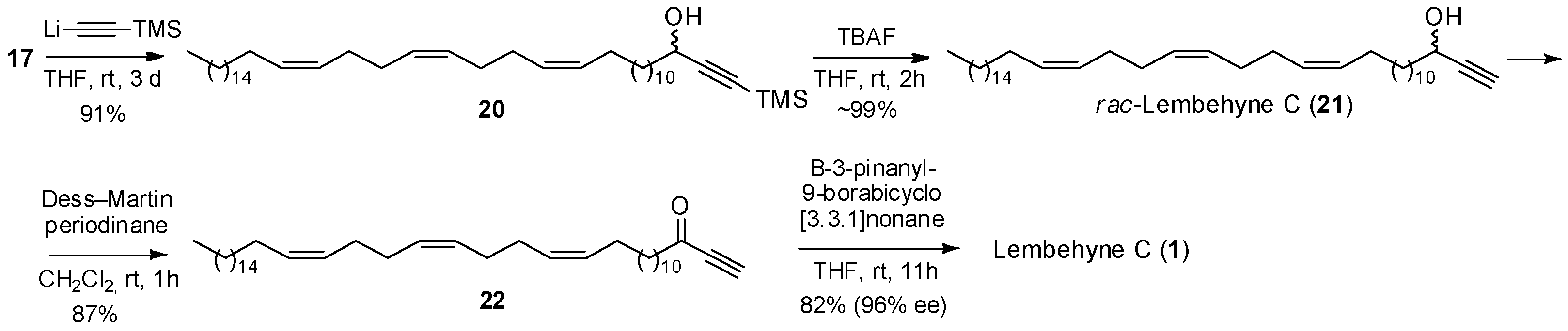
2. Results and Discussion
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newman, D.J.; Hill, R.T. New drugs from marine microbes: The tide is turning. J. Ind. Microbiol. Biotechnol. 2006, 33, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Folmer, F.; Houssen, W.E.; Scott, R.H.; Jaspars, M. Biomedical research tools from the seabed. Curr. Opin. Drug Discov. Dev. 2007, 10, 145. [Google Scholar]
- Dembitsky, V.M.; Levitsky, D.O.; Gloriozova, T.A.; Poroikov, V.V. Acetylenic Aquatic Anticancer Agents and Related Compounds. Nat. Prod. Commun. 2006, 1, 773–811. [Google Scholar] [CrossRef]
- Nathalie, L.; Fahmi, E.M.; Mohamed, M.; Philippe, A. Marine Polyacetylenes: Distribution. Biol. Prop. Synth. Stud. Nat. Prod. Chem. 2015, 45, 251–295. [Google Scholar] [CrossRef]
- Anjum, K.; Abbas, S.Q.; Ali Shah, S.A.; Akhter, N.; Batool, S.; Hassan, S.S. Marine Sponges as a Drug Treasure. Biomol. Ther. 2016, 24, 347–362. [Google Scholar] [CrossRef]
- Mayer, A.M.S.; Hamann, M.T. Marine Pharmacology in 2000: Marine Compounds with Antibacterial, Anticoagulant, Antifungal, Anti-inflammatory, Antimalarial, Antiplatelet, Antituberculosis, and Antiviral Activities; Affecting the Cardiovascular, Immune, and Nervous Systems and Other Miscellaneous Mechanisms of Action. Mar. Biotechnol. 2004, 6, 37–52. [Google Scholar]
- Zhou, Z.-F.; Menna, M.; Cai, Y.-S.; Guo, Y.-W. Polyacetylenes of marine origin: Chemistry and bioactivity. Chem. Rev. 2015, 115, 1543–1596. [Google Scholar] [CrossRef]
- Watanabe, K.; Tsuda, Y.; Hamada, M.; Omori, M.; Mori, G.; Iguchi, K.; Naoki, H.; Fujita, T.; Van Soest, R.W.M. Acetylenic Strongylodiols from a Petrosia (Strongylophora) Okinawan Marine Sponge. J. Nat. Prod. 2005, 68, 1001–1005. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Anticancer Activity of Natural and Synthetic Acetylenic Lipids. Lipids. 2006, 41, 883–924. [Google Scholar] [CrossRef]
- Aoki, S.; Matsui, K.; Tanaka, K.; Satari, R.; Kobayashi, M. Lembehyne A, a Novel Neuritogenic Polyacetylene, from a Marine Sponge of Haliclona sp. Tetrahedron 2000, 56, 9945–9948. [Google Scholar] [CrossRef]
- Aoki, S.; Matsui, K.; Takata, T.; Hong, W.; Kobayashi, M. Lembehyne A, a Spongean Polyacetylene, Induces Neuronal Differentiation in Neuroblastoma Cell. Biochem. Biophys. Res. Commun. 2001, 289, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Aoki, S.; Matsui, K.; Wei, H.; Murakami, N.; Kobayashi, M. Structure–activity relationship of neuritogenic spongean acetylene alcohols, lembehynes. Tetrahedron 2002, 58, 5417–5422. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Makarov, A.A.; Dzhemilev, U.M. Synthesis of gigantic macrocyclic polyketones through catalytic cyclometalation of cycloalkynes. Tetrahedron 2010, 66, 6685–6688. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Makarov, A.A.; Makarova, E.K.; Khalilov, L.M.; Dzhemilev, U.M. Cyclomagnesiation of N-containing 1,2-dienes with Grignard reagents catalyzed by Cp2TiCl2. J. Org. Chem. 2012, 48, 357–361. [Google Scholar]
- D’yakonov, V.A.; Makarov, A.A.; Makarova, E.K.; Tumkina, T.V.; Dzhemilev, U.M. Synthesis and transformations of metallacycles. Message 39. Zr-catalyzed cyclomagnesiation of N-containing allenes. Russ. Chem. Bull. 2012, 1, 156–162. [Google Scholar]
- D’yakonov, V.A.; Makarov, A.A.; Makarova, E.K.; Khalilov, L.M.; Dzhemilev, U.M. Synthesis and transformations of metallacycles. Message 41. Cyclomagnesiation of O-containing 1,2-dienes by Grignard reagents in the presence of Cp2TiCl2. Russ. Chem. Bull. 2012, 10, 1928–1934. [Google Scholar]
- D’yakonov, V.A.; Dzhemilev, U.M.; Makarov, A.A.; Andreev, E.N.; Dzhemileva, L.U. A short and efficient route for the synthesis of lembehyne B with neuritogenic activity. J. Org. Chem. 2016, 52, 1850–1852. [Google Scholar] [CrossRef]
- Dzhemileva, L.U.; D’yakonov, V.A.; Makarov, A.A.; Andreev, E.N.; Yunusbaeva, M.M.; Dzhemilev, U.M. The first total synthesis of the marine acetylenic alcohol, lembehyne B—A selective inducer of early apoptosis in leukemia cancer cells. Org. Biomol. Chem. 2017, 15, 470–476. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Makarov, A.A.; Dzhemileva, L.U.; Andreev, E.N.; Dzhemilev, U.M. The first total synthesis of Lembehyne B. Mendeleev Commun. 2017, 27, 122–124. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Makarov, A.A.; Dzhemileva, L.U.; Andreev, E.N.; Dzhemilev, U.M. Total Synthesis of Neuritogenic Alkynes: Lembehyne B and Key Intermediate of Lembehyne A. Chem. Select. 2017, 2, 1211–1213. [Google Scholar] [CrossRef]
- Dzhemileva, L.U.; Makarov, A.A.; Andreev, E.N.; Yunusbaeva, M.M.; Makarova, E.K.; D’yakonov, V.A.; Dzhemilev, U.M. New 1,3-Diynoic Derivatives of Natural Lembehyne B: Stereoselective Synthesis, Anticancer and Neuritogenic Activity. ACS Omega 2020, 5, 1974–1981. [Google Scholar] [CrossRef] [PubMed]
- D’yakonov, V.A.; Makarov, A.A.; Dzhemileva, L.U.; Andreev, E.N.; Makarova, E.K.; Dzhemilev, U.M. Total Synthesis of Natural Lembehyne C and Investigation of Its Cytotoxic Properties. J. Nat. Prod. 2020, 83, 2399–2409. [Google Scholar] [CrossRef]
- D’yakonov, V.A. Dzhemilev Reaction for the Synthesis of Cyclic and Acyclic Organometallic Compounds. In Organometallic Compounds: Preparation, Structure and Properties; Chin, H.F., Ed.; Nova Science Publisher: New York, NY, USA, 2010; p. 425. ISBN 978-1-60741-917-4. [Google Scholar]
- D’yakonov, V.A.; Dzhemileva, L.U.; Dzhemilev, U.M. Natural Compounds with bis-Methylene-Interrupted Z-Double Bonds: Plant Sources, Strategies of Total Synthesis, Biological Activity, and Perspectives. Phytochem. Rev. 2020, 20, 325–342. [Google Scholar] [CrossRef]
- D’yakonov, V.A. Reactions of Al- and Mg-Organic Compounds with Alkynes and 1,2-Dienes; Lap Lambert Academic Publishing: London, UK, 2015; p. 524. ISBN 978-3-659-74774-8. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makarov, A.A.; Dzhemileva, L.U.; Makarova, E.K.; Dzhemilev, U.M. Development of New Effective Methods for the Synthesis of Lembehynes A–C Exhibiting Cytotoxic and Neuritogenic Activity. Chem. Proc. 2022, 12, 19. https://doi.org/10.3390/ecsoc-26-13524
Makarov AA, Dzhemileva LU, Makarova EK, Dzhemilev UM. Development of New Effective Methods for the Synthesis of Lembehynes A–C Exhibiting Cytotoxic and Neuritogenic Activity. Chemistry Proceedings. 2022; 12(1):19. https://doi.org/10.3390/ecsoc-26-13524
Chicago/Turabian StyleMakarov, Alexey A., Lilya U. Dzhemileva, Elina Kh. Makarova, and Usein M. Dzhemilev. 2022. "Development of New Effective Methods for the Synthesis of Lembehynes A–C Exhibiting Cytotoxic and Neuritogenic Activity" Chemistry Proceedings 12, no. 1: 19. https://doi.org/10.3390/ecsoc-26-13524
APA StyleMakarov, A. A., Dzhemileva, L. U., Makarova, E. K., & Dzhemilev, U. M. (2022). Development of New Effective Methods for the Synthesis of Lembehynes A–C Exhibiting Cytotoxic and Neuritogenic Activity. Chemistry Proceedings, 12(1), 19. https://doi.org/10.3390/ecsoc-26-13524






