Structure-Property Influence on the Amphiphilicity of Phenolipids †
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Synthesis of Phenolic Acid Esters
2.3. Calculation of Partition Coefficients (log P) for Phenolic Compounds
2.3.1. Shake-Flask Method
2.3.2. Theoretical Calculation
2.4. Statistical Analysis
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Menezes, J.C.; Kamat, S.P.; Cavaleiro, J.A.S.; Gaspar, A.; Garrido, J.; Borges, F. Synthesis and Antioxidant Activity of Long Chain Alkyl Hydroxycinnamates. Eur. J. Med. Chem. 2011, 46, 773–777. [Google Scholar] [CrossRef] [PubMed]
- Rabiej-Kozioł, D.; Roszek, K.; Krzemiński, M.P.; Szydłowska-Czerniak, A. Phenolipids as New Food Additives: From Synthesis to Cell-Based Biological Activities. Food Addit. Contam. Part A 2022, 39, 1365–1379. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, G.; Suzuka, C.; Shoji, A.; Shibusawa, Y.; Yanagida, A. High-Throughput Determination of Octanol/Water Partition Coefficients Using a Shake-Flask Method and Novel Two-Phase Solvent System. J. Pharm. Biomed. Anal. 2016, 117, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Esteves, M.; Siquet, C.; Gaspar, A.; Rio, V.; Sousa, J.B.; Reis, S.; Marques, M.P.M.; Borges, F. Antioxidant Versus Cytotoxic Properties of Hydroxycinnamic Acid Derivatives—A New Paradigm in Phenolic Research. Arch. Pharm. Chem. Life Sci. 2008, 341, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.K.; Singhvi, G.; Tyagi, A.; Agarwal, H.; Krishna, K.V. Spectrophotometric Determination of PKa and Log P of Risperidone. J. Appl. Pharm. Sci. 2017, 7, 155–158. [Google Scholar] [CrossRef][Green Version]
- OECD. 107:1995; Guideline for The Testing of Chemicals; Partition Coefficient (n-octanol/water): Shake Flask Method; OECD: Paris, France, 1995. [Google Scholar]
- Gaspar, A.; Martins, M.; Silva, P.; Garrido, E.M.; Garrido, J.; Firuzi, O.; Miri, R.; Saso, L.; Borges, F. Dietary Phenolic Acids and Derivatives. Evaluation of the Antioxidant Activity of Sinapic Acid and Its Alkyl Esters. J. Agric. Food Chem. 2010, 5, 11273–11280. [Google Scholar] [CrossRef] [PubMed]
- Garrido, J.; Gaspar, A.; Garrido, E.M.; Miri, R.; Tavakkoli, M.; Pourali, S.; Saso, L.; Borges, F.; Firuzi, O. Alkyl Esters of Hydroxycinnamic Acids with Improved Antioxidant Activity and Lipophilicity Protect PC12 Cells against Oxidative Stress. Biochimie 2012, 94, 961–967. [Google Scholar] [CrossRef] [PubMed]
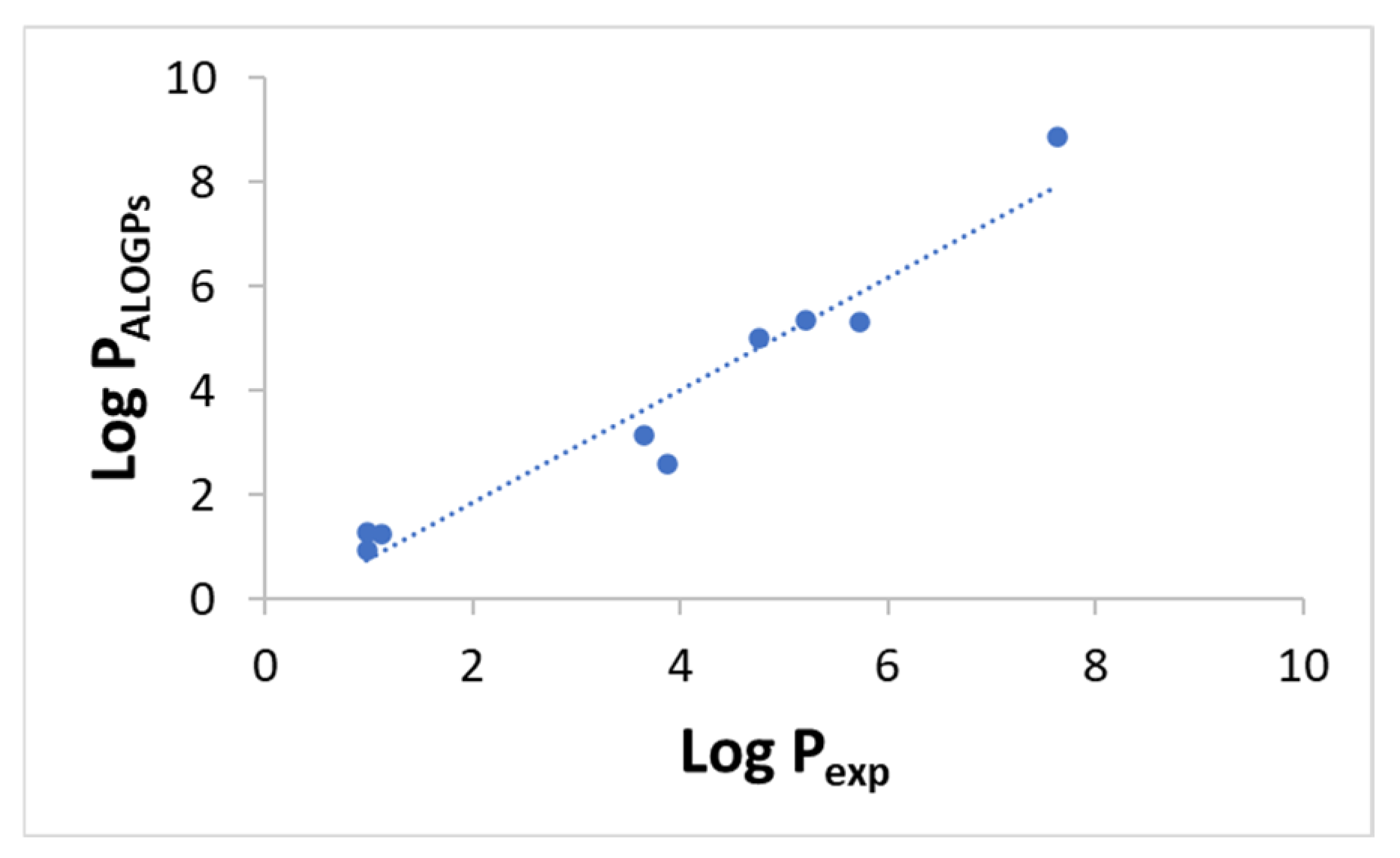
| Phenolic Compound | Log Pexp ± SD | Log PALOGPs |
|---|---|---|
| SA | 0.98 ± 0.05 | 1.26 |
| ESA | 3.87 ± 0.13 | 2.60 |
| OSA | 5.20 ± 0.24 | 5.34 |
| CSA | 7.63 ± 0.39 | 8.87 |
| CA | 0.99 ± 0.12 | 0.94 |
| OCA | 4.75 ± 0.05 | 5.02 |
| FA | 1.12 ± 0.07 | 1.25 |
| OFA | 5.72 ± 0.04 | 5.32 |
| BHA | 3.64 ± 0.05 | 3.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabiej-Kozioł, D.; Kryska, N.; Szydłowska-Czerniak, A. Structure-Property Influence on the Amphiphilicity of Phenolipids. Chem. Proc. 2022, 12, 17. https://doi.org/10.3390/ecsoc-26-13533
Rabiej-Kozioł D, Kryska N, Szydłowska-Czerniak A. Structure-Property Influence on the Amphiphilicity of Phenolipids. Chemistry Proceedings. 2022; 12(1):17. https://doi.org/10.3390/ecsoc-26-13533
Chicago/Turabian StyleRabiej-Kozioł, Dobrochna, Natalia Kryska, and Aleksandra Szydłowska-Czerniak. 2022. "Structure-Property Influence on the Amphiphilicity of Phenolipids" Chemistry Proceedings 12, no. 1: 17. https://doi.org/10.3390/ecsoc-26-13533
APA StyleRabiej-Kozioł, D., Kryska, N., & Szydłowska-Czerniak, A. (2022). Structure-Property Influence on the Amphiphilicity of Phenolipids. Chemistry Proceedings, 12(1), 17. https://doi.org/10.3390/ecsoc-26-13533







