Exploring BINOL-O-BODIPY-Based Dimers as Advanced Chromophoric Platforms Enabling Triplet State Population †
Abstract
1. Introduction
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Synthesis
4.2. Photophysical Properties
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, J.; Wu, W.; Sun, J.; Guo, S. Triplet Photosensitizers: From Molecular Design to Applications. Chem. Soc. Rev. 2013, 42, 5323–5351. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Hou, Y.; Liu, L.; Zhao, J. Triplet Photosensitizers Showing Strong Absorption of Visible Light and Long-Lived Triplet Excited States and Application in Photocatalysis: A Mini Review. Energy Fuels 2021, 35, 18942–18956. [Google Scholar] [CrossRef]
- Pang, E.; Zhao, S.; Wang, B.; Niu, G.; Song, X.; Lan, M. Strategies to Construct Efficient Singlet Oxygen-Generating Photosensitizers. Coord. Chem. Rev. 2022, 472, 214780. [Google Scholar] [CrossRef]
- Mahammed, A.; Gross, Z. Corroles as Triplet Photosensitizers. Coord. Chem. Rev. 2019, 379, 121–132. [Google Scholar] [CrossRef]
- Schäferling, M. The Art of Fluorescence Imaging with Chemical Sensors. Angew. Chem. Int. Ed. 2012, 51, 3532–3554. [Google Scholar] [CrossRef] [PubMed]
- Boens, N.; Verbelen, B.; Ortiz, M.J.; Jiao, L.; Dehaen, W. Synthesis of BODIPY Dyes through Postfunctionalization of the Boron Dipyrromethene Core. Coord. Chem. Rev. 2019, 399, 213024. [Google Scholar] [CrossRef]
- Bañuelos, J. BODIPY Dye, the Most Versatile Fluorophore Ever? Chem. Rec. 2016, 16, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Dong, Y.; Zhao, X.; Imran, M.; Tang, G.; Zhao, J.; Liu, Q. Bodipy Derivatives as Triplet Photosensitizers and the Related Intersystem Crossing Mechanisms. Front. Chem. 2019, 7, 821. [Google Scholar] [CrossRef]
- Bassan, E.; Gualandi, A.; Cozzi, P.G.; Ceroni, P. Design of BODIPY Dyes as Triplet Photosensitizers: Electronic Properties Tailored for Solar Energy Conversion, Photoredox Catalysis and Photodynamic Therapy. Chem. Sci. 2021, 12, 6607–6628. [Google Scholar] [CrossRef] [PubMed]
- Kamkaew, A.; Lim, S.H.; Lee, H.B.; Kiew, L.V.; Chung, L.Y.; Burgess, K. BODIPY Dyes in Photodynamic Therapy. Chem. Soc. Rev. 2012, 42, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhang, R.; Zhang, X.-F.; Liu, J.; Luo, L. Halogenated BODIPY Photosensitizers: Photophysical Processes for Generation of Excited Triplet State, Excited Singlet State and Singlet Oxygen. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 272, 120965. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, Y.; Kolemen, S.; Duman, S.; Dede, Y.; Dolen, Y.; Kilic, B.; Kostereli, Z.; Yildirim, L.T.; Dogan, A.L.; Guc, D.; et al. Designing Excited States: Theory-Guided Access to Efficient Photosensitizers for Photodynamic Action. Angew. Chem. Int. Ed. 2011, 50, 11937–11941. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Yin, Z.; Ding, K.; Tang, Q.; Li, J.; Si, W.; Shao, J.; Zhang, Q.; Huang, W.; Dong, X. BODIPY Derivatives for Photodynamic Therapy: Influence of Configuration versus Heavy Atom Effect. ACS Appl. Mater. Interfaces 2017, 9, 32475–32481. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-G.; Zhang, L.-N.; Li, Q.; Yang, Y.; Wu, Y.; Fan, X.; Song, M.; Kuang, G.-C. Dimeric BODIPYs with Different Linkages: A Systematic Investigation on Structure-Properties Relationship. Tetrahedron 2017, 73, 6894–6900. [Google Scholar] [CrossRef]
- Gartzia-Rivero, L.; Sánchez-Carnerero, E.M.; Jiménez, J.; Bañuelos, J.; Moreno, F.; Maroto, B.L.; López-Arbeloa, I.; de la Moya, S. Modulation of ICT Probability in Bi(Polyarene)-Based O-BODIPYs: Towards the Development of Low-Cost Bright Arene-BODIPY Dyads. Dalton Trans. 2017, 46, 11830–11839. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, J.; Prieto-Montero, R.; Maroto, B.L.; Moreno, F.; Ortiz, M.J.; Oliden-Sánchez, A.; López-Arbeloa, I.; Martínez-Martínez, V.; de la Moya, S. Manipulating Charge-Transfer States in BODIPYs: A Model Strategy to Rapidly Develop Photodynamic Theragnostic Agents. Chem. Eur. J. 2020, 26, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, J.; Prieto-Montero, R.; Serrano, S.; Stachelek, P.; Rebollar, E.; Maroto, B.L.; Moreno, F.; Martinez-Martinez, V.; Pal, R.; García-Moreno, I.; et al. BINOL blocks as accessible triplet state modulators in BODIPY dyes. Chem. Commun. 2022, 58, 6385–6388. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Gu, W.; Yu, C.; Lv, X.; Wang, H.; Hao, E.; Jiao, L. Syntheses and Photophysical Properties of meso-Phenylene ridged Boron Dipyrromethene Monomers, Dimers and Trimer. Chin. J. Chem. 2016, 34, 989–996. [Google Scholar] [CrossRef]
- Prieto-Montero, R.; Sola-Llano, R.; Montero, R.; Longarte, A.; Arbeloa, T.; López-Arbeloa, I.; Martínez-Martínez, V.; Lacombe, S. Methylthio BODIPY as a standard triplet photosensitizer for singlet oxygen production: A photophysical study. Phys. Chem. Chem. Phys. 2019, 21, 20403–20414. [Google Scholar] [CrossRef] [PubMed]

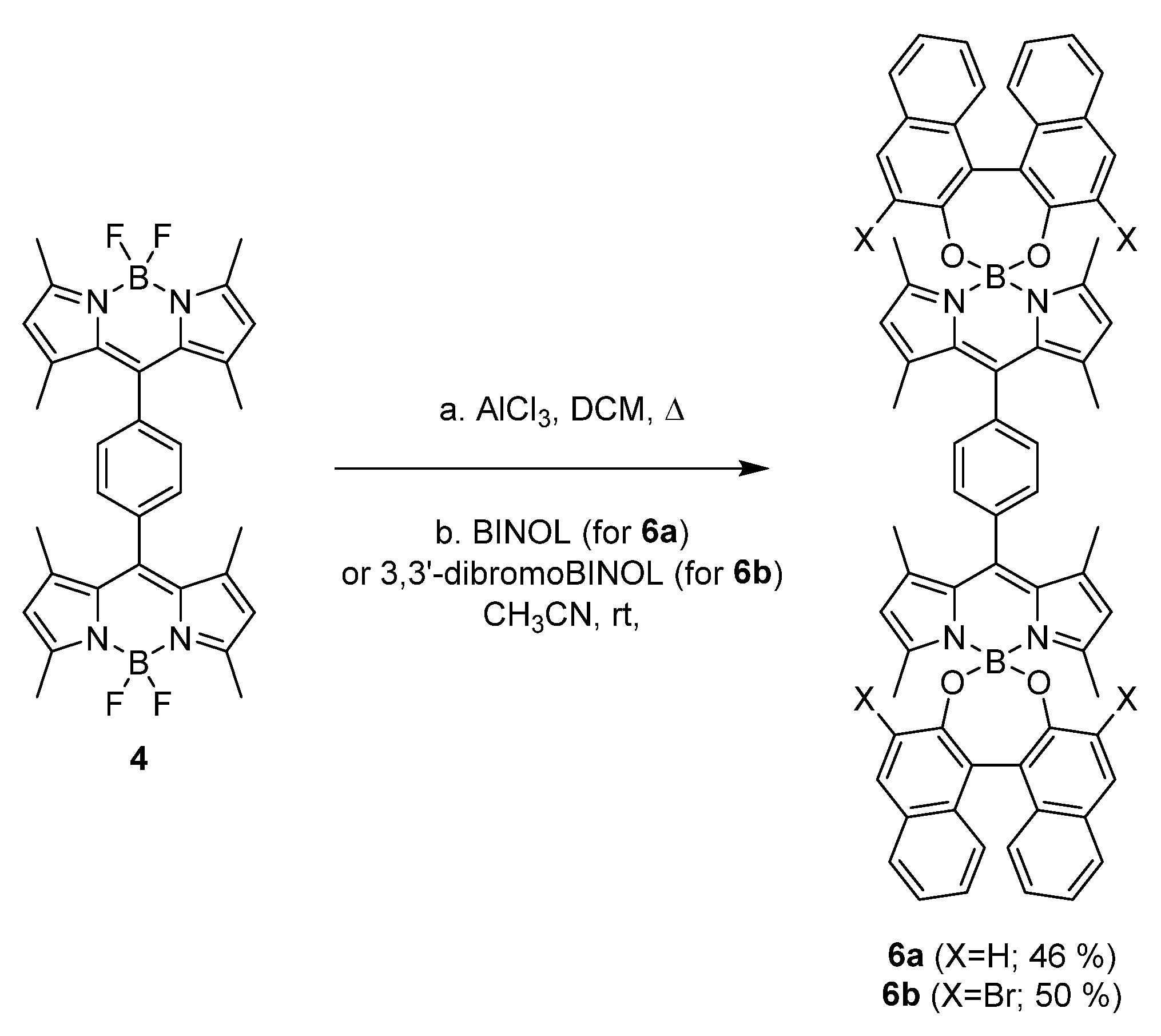
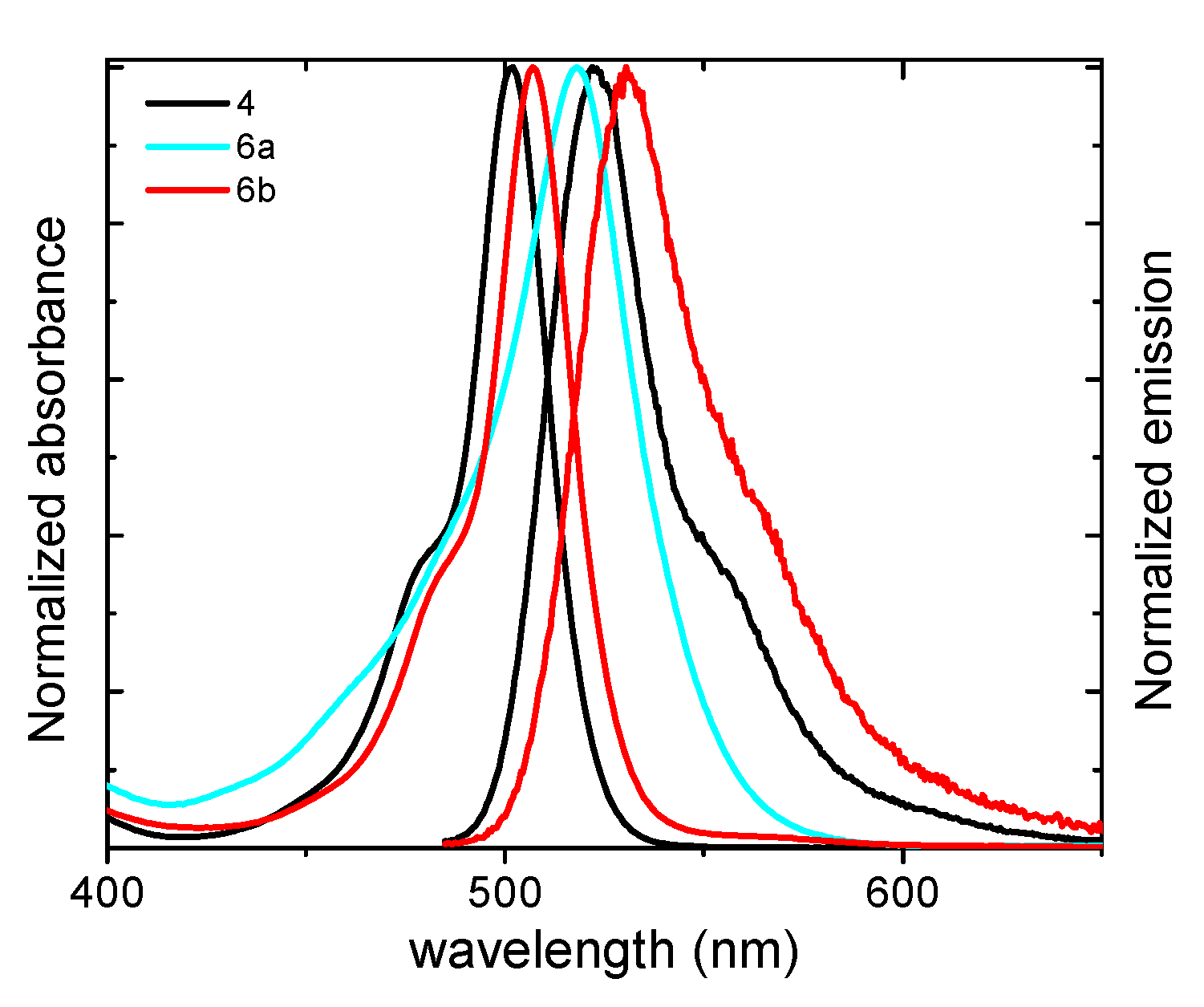
| Compound | λab (nm) | εmax (104 M−1 cm−1) | λfl (nm) | ϕfl | τ (ns) | ϕΔ |
|---|---|---|---|---|---|---|
| 4 | 502.0 | 5.9 | 522.0 | 0.39 | 3.86 | 0.05 |
| 6a | 518.0 | 8.7 | 530.0 | 0.00 | - | 0.03 |
| 6b | 507.0 | 5.1 | 530.5 | 0.06 | 0.73 (99%) 2.37 (1%) | 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano-Buitrago, S.; Jiménez, J.; Prieto-Montero, R.; Moreno, F.; Martínez-Martínez, V.; Maroto, B.L.; de la Moya, S. Exploring BINOL-O-BODIPY-Based Dimers as Advanced Chromophoric Platforms Enabling Triplet State Population. Chem. Proc. 2022, 12, 15. https://doi.org/10.3390/ecsoc-26-13671
Serrano-Buitrago S, Jiménez J, Prieto-Montero R, Moreno F, Martínez-Martínez V, Maroto BL, de la Moya S. Exploring BINOL-O-BODIPY-Based Dimers as Advanced Chromophoric Platforms Enabling Triplet State Population. Chemistry Proceedings. 2022; 12(1):15. https://doi.org/10.3390/ecsoc-26-13671
Chicago/Turabian StyleSerrano-Buitrago, Sergio, Josué Jiménez, Ruth Prieto-Montero, Florencio Moreno, Virginia Martínez-Martínez, Beatriz L. Maroto, and Santiago de la Moya. 2022. "Exploring BINOL-O-BODIPY-Based Dimers as Advanced Chromophoric Platforms Enabling Triplet State Population" Chemistry Proceedings 12, no. 1: 15. https://doi.org/10.3390/ecsoc-26-13671
APA StyleSerrano-Buitrago, S., Jiménez, J., Prieto-Montero, R., Moreno, F., Martínez-Martínez, V., Maroto, B. L., & de la Moya, S. (2022). Exploring BINOL-O-BODIPY-Based Dimers as Advanced Chromophoric Platforms Enabling Triplet State Population. Chemistry Proceedings, 12(1), 15. https://doi.org/10.3390/ecsoc-26-13671







