Impact of Water Deficit on Primary Metabolism at the Whole Plant Level in Bread Wheat Grown under Elevated CO2 and High Temperature at Different Developmental Stages †
Abstract
1. Introduction
2. Methods
2.1. Plant Material and Growing Conditions
2.2. Sampling Procedure
2.3. C-N Metabolites Quantification
2.4. Determination of Biomass Parameters
2.5. Statistical Analyses
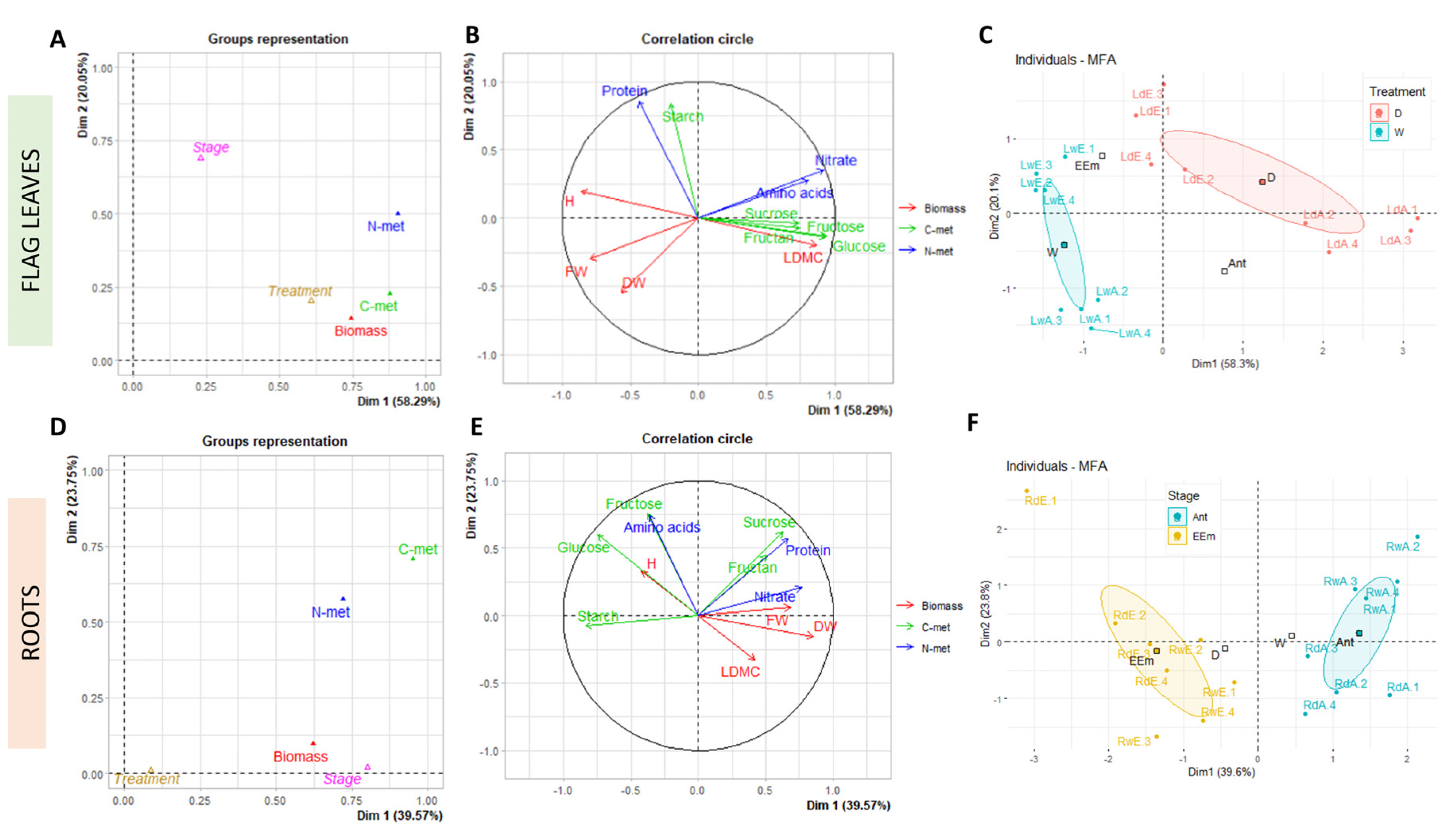
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asseng, S.; Foster, I.; Turner, N.C. The impact of temperature variability on wheat yields. Glob. Chang. Biol. 2011, 17, 997–1012. [Google Scholar] [CrossRef]
- FAO. How to Feed the World in 2050. High-Level Experts Forum 2009. Available online: https://www.fao.org/fileadmin/templates/wsfs/docs/expert_paper/How_to_Feed_the_World_in_2050.pdf (accessed on 30 December 2021).
- Porter, J.R.; Semenov, M.A. Crop responses to climatic variation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005, 360, 2021–2035. [Google Scholar] [CrossRef] [PubMed]
- Weisheimer, A.; Palmer, T.N. Changing frequency of occurrence of extreme seasonal temperatures under global warming. Geophys. Res. Lett. 2005, 32, L20721. [Google Scholar] [CrossRef]
- Semenov, M.A.; Shewry, P.R. Modelling predicts that heat stress, not drought, will increase vulnerability of wheat in Europe. Sci. Rep. 2011, 1, 66. [Google Scholar] [CrossRef]
- Lobell, B.D.; Sibley, A.; Ortiz-Monasterio, J.I. Extreme heat effects on wheat senescence in India. Nat. Clim. Chang. 2012, 2, 186–189. [Google Scholar] [CrossRef]
- Asseng, S.; Ewert, F.; Martre, P.; Rötter, R.P.; Lobell, D.B.; Cammarano, D.; Kimball, B.A.; Ottman, M.J.; Wall, G.W.; White, J.W.; et al. Rising temperatures reduce global wheat production. Nat. Clim. Chang. 2015, 5, 143–147. [Google Scholar] [CrossRef]
- Barnabas, B.; Jäger, K.; Fehér, A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2008, 31, 11–38. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, M.; Siddique, K.H.M. Drought stress in wheat during flowering and grain-filling periods. CRC Crit. Rev. Plant Sci. 2014, 33, 331–349. [Google Scholar] [CrossRef]
- Senapati, N.; Stratonovitch, P.; Paul, M.J.; Semenov, M.A. Drought tolerance during reproductive development is important for increasing wheat yield potential under climate change in Europe. J. Exp. Bot. 2019, 70, 2549–2560. [Google Scholar] [CrossRef]
- Izanloo, A.; Condon, A.G.; Langridge, P.; Tester, M.; Schnurbusch, T. Different mechanisms of adaptation to cyclic water stress in two South Australian bread wheat cultivars. J. Exp. Bot. 2008, 59, 3327–3346. [Google Scholar] [CrossRef]
- Comas, L.H.; Becker, S.R.; Cruz, V.M.; Byrne, P.F.; Dierig, D.A. Root traits contributing to plant productivity under drought. Front. Plant Sci. 2013, 4, 442. [Google Scholar] [CrossRef] [PubMed]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sabia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [PubMed]
- Steudle, E. Water uptake by roots: Effects of water deficit. J. Exp. Bot. 2000, 51, 1531–1542. [Google Scholar] [CrossRef] [PubMed]
- Rizhsky, L.; Liang, H.; Mittler, R. The combined effect of drought stress and heat shock on gene expression in tobacco. Plant Physiol. 2002, 130, 1143–1151. [Google Scholar] [CrossRef]
- Mahajan, S.; Tuteja, N. Cold, salinity and drought stresses: An overview. Arch. Biochem. Biophys. 2005, 444, 139–158. [Google Scholar] [CrossRef]
- Monneveux, P.; Rekika, D.; Acevedo, E.; Merah, O. Effect of drought on leaf gas exchange, carbon isotope discrimination, transpiration efficiency and productivity in field grown durum wheat genotypes. Plant Sci. 2006, 170, 867–872. [Google Scholar] [CrossRef]
- Slama, I.; Abdelly, C.; Bouchereau, A.; Flower, T.; Savouré, A. Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann. Bot. 2015, 115, 433–447. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Medina, S.; Vicente, R.; Amador, A.; Araus. J.L. Interactive effects of elevated [CO2] and water stress on physiological traits and gene expression during vegetative growth in four durum wheat genotypes. Front. Plant Sci. 2007, 7, 1738. [Google Scholar]
- Kumar, M.; Kumar-Patel, M.; Kumar, N.; Bajpai, A.B.; Siddique, K.H.M. Metabolomics and molecular approaches reveal drought stress tolerance in plants. Int. J. Mol. Sci. 2021, 22, 9108. [Google Scholar] [CrossRef]
- Vicente, R.; Pérez, P.; Martínez-Carrasco, R.; Usadel, B.; Kostadinova, S.; Morcuende, R. Quantitative RT-PCR platform to measure transcript levels of C and N metabolism-related genes in durum wheat: Transcript profiles in elevated [CO2] and high temperature at different levels of N supply. Plant Cell Physiol. 2015, 56, 1556–1573. [Google Scholar] [CrossRef] [PubMed]
- Zadoks, J.C.; Chang, T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Morcuende, R.; Kostadinova, S.; Pérez, P.; Martín del Molino, I.M.; Martínez-Carrasco, R. Nitrate is a negative signal for fructan synthesis, and the fructosyltransferase-inducing trehalose inhibits nitrogen and carbon assimilation in excised barley leaves. New Phytol. 2004, 161, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Vicente, R.; Pérez, P.; Martínez-Carrasco, R.; Feil, R.; Lunn, J.E.; Watanabe, M.; Arrivault, S.; Stitt, M.; Hoefgen, R.; Morcuende, R. Metabolic and transcriptional analysis of durum wheat responses to elevated CO2 at low and high nitrate supply. Plant Cell Physiol. 2016, 57, 2133–2146. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Barbero, E.L.; Pérez, P.; Martínez-Carrasco, R.; Arellano, J.B.; Morcuende, R. Screening for higher grain yield and biomass among sixty bread wheat genotypes grown under elevated CO2 and high-temperature conditions. Plants 2021, 10, 1596. [Google Scholar] [CrossRef] [PubMed]
- Noleto Luz Pequeno, D.; Hernandez-Ochoa, I.M.; Reynolds, M.; Sonder, K.; Molero-Milan, A.; Robertson, R.; Lopes, M.S.; Xiong, W.; Kropff, M.; Asseng, S.; et al. Climate impact and adaptation to heat and drought stress of regional and global wheat production. Environ. Res. Lett. 2021, 16, 054070. [Google Scholar] [CrossRef]
- Boudiar, R.; Casas, A.M.; Gioia, T.; Fiorani, F.; Nagel, K.A.; Igartua, E. Effects of low water availability on root placement and shoot development in landraces and modern barley cultivars. Agronomy 2020, 10, 134. [Google Scholar] [CrossRef]
- Guo, R.; Shi, L.; Jiao, Y.; Li, M.; Zhong, X.; Gu, F.; Liu, Q.; Xia, X.; Li, H. Metabolic responses to drought stress in the tissues of drought-tolerant and drought-sensitive wheat genotype seedlings. AoB Plants 2018, 10, ply016. [Google Scholar] [CrossRef]
- Goggin, D.E.; Setter, T.L. Fructosyltransferase activity and fructan accumulation during development in wheat exposed to terminal drought. Funct. Plant Biol. 2004, 31, 11–21. [Google Scholar] [CrossRef]
- Wardlaw, I.F.; Willenbrink, J. Mobilization of fructan reserves and changes in enzyme activities in wheat stems correlate with water stress during kernel filling. New Phytol. 2000, 148, 413–422. [Google Scholar] [CrossRef]
- Bowne, J.B.; Erwin, T.A.; Juttner, J.; Schnurbusch, T.; Langridge, P.; Bacic, A.; Roessner, U. Drought responses of leaf tissues from wheat cultivars of differing drought tolerance at the metabolite level. Mol. Plant 2012, 5, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, F.A.; Golovina, E.A.; Buitink, J. Mechanisms of plant desiccation tolerance. Trends Plant Sci. 2001, 6, 431–438. [Google Scholar] [CrossRef]
- Anjum, S.A.; Xie, X.Y.; Wang, L.C.; Saleem, M.F.; Man, C.; Lei, W. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011, 6, 2026–2032. [Google Scholar]
- Michaletti, A.; Naghavi, M.R.; Toorchi, M.; Zolla, L.; Rinalducci, S. Metabolomics and proteomics reveal drought-stress responses of leaf tissues from spring-wheat. Sci. Rep. 2018, 8, 5710. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Apodaca, J.; Agirresarobe, A.; Martínez-Goñi, X.S.; Yoldi-Achalandabaso, A.; Pérez-López, U. N metabolism performance in Chenopodium quinoa subjected to drought or salt stress conditions. Plant Physiol. Biochem. 2020, 155, 725–734. [Google Scholar] [CrossRef]
- Oaks, A.; Hirel, B. Nitrogen metabolism in roots. Annu. Rev. Plant Physiol. 1985, 36, 345–365. [Google Scholar] [CrossRef]
- Barillot, R.; Chambon, C.; Andrieu, B. CN-Wheat, a functional–structural model of carbon and nitrogen metabolism in wheat culms after anthesis. II. Model evaluation. Ann. Bot. 2016, 118, 1015–1031. [Google Scholar] [CrossRef][Green Version]
- Shao, K.; Bai, Z.; Li, M.; Yu, C.; Shao, J.; Sun, Y.; Li, G.; Zhang, S.; Wang, R. Sucrose metabolism enzymes affect sucrose content rather than root weight in sugar beet (Beta vulgaris) at different growth stages. Sugar Tech. 2020, 22, 504–517. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bueno-Ramos, N.; González-Hernández, A.I.; Marcos-Barbero, E.L.; Miranda-Apodaca, J.; Bendou, O.; Gutiérrez-Fernández, I.; Arellano, J.B.; Morcuende, R. Impact of Water Deficit on Primary Metabolism at the Whole Plant Level in Bread Wheat Grown under Elevated CO2 and High Temperature at Different Developmental Stages. Chem. Proc. 2022, 10, 6. https://doi.org/10.3390/IOCAG2022-12232
Bueno-Ramos N, González-Hernández AI, Marcos-Barbero EL, Miranda-Apodaca J, Bendou O, Gutiérrez-Fernández I, Arellano JB, Morcuende R. Impact of Water Deficit on Primary Metabolism at the Whole Plant Level in Bread Wheat Grown under Elevated CO2 and High Temperature at Different Developmental Stages. Chemistry Proceedings. 2022; 10(1):6. https://doi.org/10.3390/IOCAG2022-12232
Chicago/Turabian StyleBueno-Ramos, Nara, Ana I. González-Hernández, Emilio L. Marcos-Barbero, Jon Miranda-Apodaca, Ouardia Bendou, Ismael Gutiérrez-Fernández, Juan B. Arellano, and Rosa Morcuende. 2022. "Impact of Water Deficit on Primary Metabolism at the Whole Plant Level in Bread Wheat Grown under Elevated CO2 and High Temperature at Different Developmental Stages" Chemistry Proceedings 10, no. 1: 6. https://doi.org/10.3390/IOCAG2022-12232
APA StyleBueno-Ramos, N., González-Hernández, A. I., Marcos-Barbero, E. L., Miranda-Apodaca, J., Bendou, O., Gutiérrez-Fernández, I., Arellano, J. B., & Morcuende, R. (2022). Impact of Water Deficit on Primary Metabolism at the Whole Plant Level in Bread Wheat Grown under Elevated CO2 and High Temperature at Different Developmental Stages. Chemistry Proceedings, 10(1), 6. https://doi.org/10.3390/IOCAG2022-12232







