Effect of Exogenous Application of an Aqueous Quercetin Solution on the Physiological Properties of Andropogon gerardi Plants †
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Gas Exchange Measurement
3.2. Chlorophyll Fluorescence
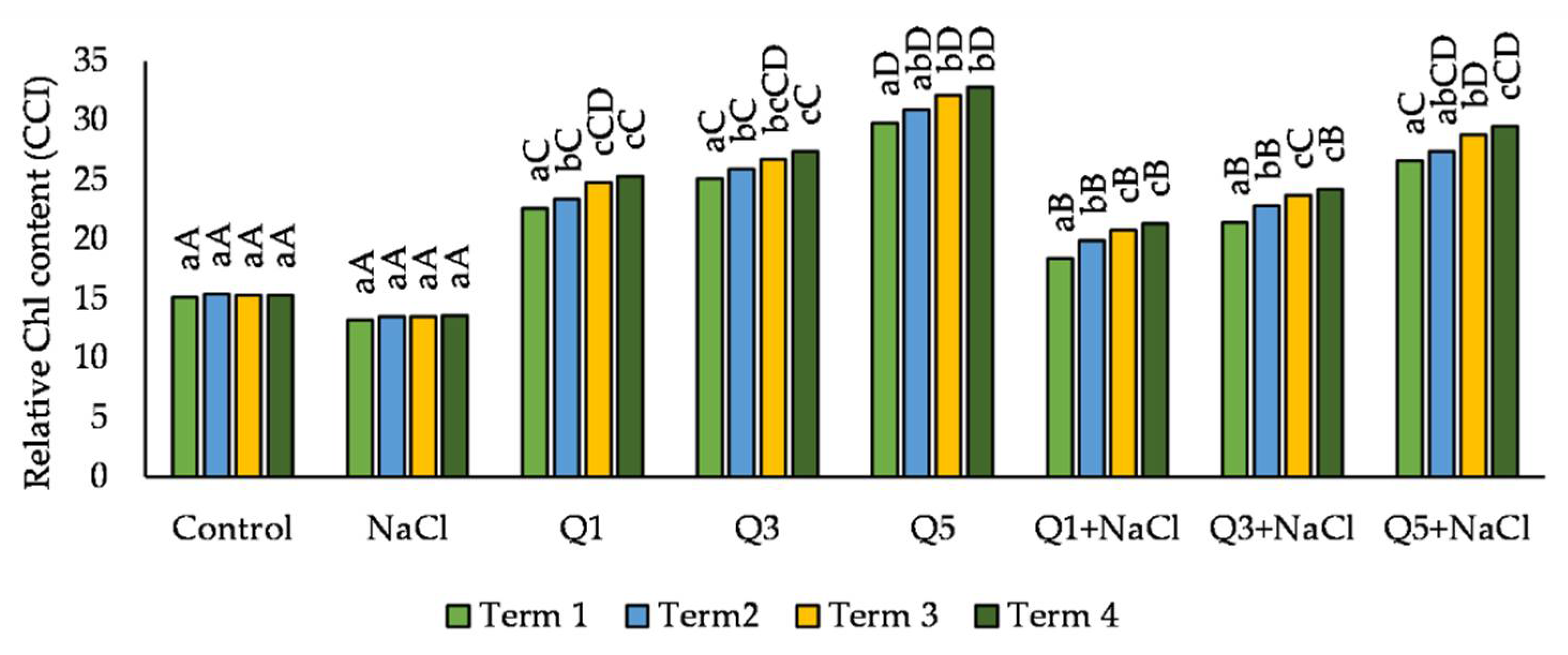
3.3. Relative Chlorophyll Content
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dien, B.S.; Jung, H.J.G.; Vogel, K.P.; Casler, M.D.; Lamb, J.F.S.; Iten, L.; Mitchell, R.B.; Sarath, G. Chemical composition and response to dilute-acid pretreatment and enzymatic saccharification of alfalfa, reed canarygrass, and switchgrass. Biomass Bioenergy 2006, 30, 880–891. [Google Scholar] [CrossRef] [Green Version]
- Gan, J.; Yuan, W.; Johnson, L.; Wang, D.; Nelson, R.; Zhang, K. Hydrothermal conversion of big bluestem for bio-oil production: The effect of ecotype and planting location. Bioresour. Technol. 2021, 116, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Johnson, L.; Prasad, V.P.V.; Pei, Z.; Wang, D. Big bluestem as a bioenergy crop: A review. Renew. Sust. Energ. Rev. 2015, 52, 740–756. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, J.C. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 2004, 166, 3–16. [Google Scholar] [CrossRef]
- Hussain, K.; Majeed, A.; Nawaz, K.; Khizar, H.B.; Nisar, M.F. Effect of different levels of salinity on growth and ion contents of black seeds (Nigella sativa L.). Curr. Res. J. Biol. Sci. 2009, 1, 135–138. [Google Scholar]
- Ferrante, A.; Mariani, L. Agronomic management for enhancing plant tolerance to abiotic stresses: High and low values of temperature, light intensity, and relative humidity. Horticulturae 2018, 4, 21. [Google Scholar] [CrossRef] [Green Version]
- Zandalinas, S.I.; Mittler, R.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A. Plant adaptations to the combination of drought and high temperatures. Physiol. Plant. 2018, 162, 2–12. [Google Scholar] [CrossRef] [Green Version]
- Mariani, L.; Ferrante, A. Agronomic management for enhancing plant tolerance to abiotic stresses—drought, salinity, hypoxia, and lodging. Horticulturae 2017, 3, 52. [Google Scholar] [CrossRef] [Green Version]
- Stojaković, M.; Mitrović, B.; Zorić, M. Grouping pattern of maize test locations and its impact on hybrid zoning. Euphytica 2015, 204, 419–431. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, E.; Moreno, D.A.; Ferreres, F.; Mar Rubio-Wilhelmi, M.D.; Ruiz, J.M. Differential responses of five cherry tomato varieties to water stress: Changes on phenolic metabolites and related enzymes. Phytochemistry 2011, 72, 723–729. [Google Scholar] [CrossRef]
- Singh, P.; Arif, Y.; Bajguz, A.; Hayat, S. The role of quercetin in plants. Plant Physiol. Biochem. 2021, 166, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Dobrikova, A.G.; Apostolova, E.L. Damage and protection of the photosynthetic apparatus from UV-Bradiation. II. Effect of quercetin at different pH. J. Plant Physiol. 2015, 184, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Migut, D.; Jańczak-Pieniążek, M.; Piechowiak, T.; Buczek, J.; Balawejder, M. Physiological Response of Maize Plants (Zea mays L.) to the Use of the Potassium Quercetin Derivative. Int. J. Mol. Sci. 2021, 22, 7384. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A. Understanding and improving global crop response to ozone pollution. Plant J. 2016, 90, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, S.; Tomassetti, M.; Caratu, M.R. Quercetin reduces chromosome aberrations induced by atrazine in the Allium cepa test. Environ. Mol. Mutagen. 2006, 47, 254–259. [Google Scholar] [CrossRef]
- Shah, A.; Smith, D.L. Flavonoids in Agriculture: Chemistry and Roles in, Biotic and Abiotic Stress Responses, and Microbial Associations. Agronomy 2020, 10, 1209. [Google Scholar] [CrossRef]
- Dann, M.S.; Pell, E.J. Decline of Activity and Quantity of Ribulose Bisphosphate Carboxylase/Oxygenase and Net Photosynthesis in Ozone-Treated Potato Foliage. Plant Physiol. 1989, 91, 427–432. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Cetner, M.D.; Dąbrowski, P.; Samborska, I.A.; Łukasik, I.; Swoczyna, T.; Pietkiewicz, S.; Bąba, W. Chlorophyll fluorescence measurements in environmental studies. Kosmos 2016, 65, 197–205. Available online: http://psjd.icm.edu.pl/psjd/element/bwmeta1.element.bwnjournal-article-ksv65p197kz (accessed on 25 November 2021). (In Polish).
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [Green Version]
- Rady, M.M.; Taha, R.S.; Mahdi, A.H.A. Proline enhances growth, productivity and anatomy of two varieties of Lupinus terms L. grown under salt stress. S. Afr. J. Bot. 2016, 102, 221–227. [Google Scholar] [CrossRef]
- Dawood, M.G.; Taie, H.A.A.; Nassar, R.M.A.; Abdelhamid, M.T.; Schmidhalter, U. The changes induced in the physiological; biochemical and anatomical characteristics of Vicia faba by the exogenous application of proline under seawater stress. S. Afr. J. Bot. 2014, 93, 54–63. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Migut, D.; Jańczak-Pieniążek, M.; Piechowiak, T.; Skrobacz, K. Effect of Exogenous Application of an Aqueous Quercetin Solution on the Physiological Properties of Andropogon gerardi Plants. Chem. Proc. 2022, 10, 23. https://doi.org/10.3390/IOCAG2022-12341
Migut D, Jańczak-Pieniążek M, Piechowiak T, Skrobacz K. Effect of Exogenous Application of an Aqueous Quercetin Solution on the Physiological Properties of Andropogon gerardi Plants. Chemistry Proceedings. 2022; 10(1):23. https://doi.org/10.3390/IOCAG2022-12341
Chicago/Turabian StyleMigut, Dagmara, Marta Jańczak-Pieniążek, Tomasz Piechowiak, and Karol Skrobacz. 2022. "Effect of Exogenous Application of an Aqueous Quercetin Solution on the Physiological Properties of Andropogon gerardi Plants" Chemistry Proceedings 10, no. 1: 23. https://doi.org/10.3390/IOCAG2022-12341
APA StyleMigut, D., Jańczak-Pieniążek, M., Piechowiak, T., & Skrobacz, K. (2022). Effect of Exogenous Application of an Aqueous Quercetin Solution on the Physiological Properties of Andropogon gerardi Plants. Chemistry Proceedings, 10(1), 23. https://doi.org/10.3390/IOCAG2022-12341






