Abscisic Acid as a Marker of Metabolic Imbalance: Serum Levels from Diabetic and Smoking Subjects
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Sample Collection and Metabolic Profiling
2.3. Quantification of Abscisic Acid (ABA)
2.4. Statistical Analysis
3. Results
3.1. Baseline Demographic and Metabolic Characteristics
3.2. Progressive Decline of ABA from Healthy to Diabetic and Smoking Individuals
3.3. Higher Tobacco Exposure Is Associated with Lower Circulating ABA
4. Discussion
5. Strengths and Limits of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABA | Abscisic acid |
| T2DM | Type 2 diabetes mellitus |
| FPG | Fasting plasma glucose |
| TC | Total cholesterol |
| LDL-C | Low-density lipoprotein cholesterol |
| HDL-C | High-density lipoprotein cholesterol |
| TG | Triglycerides |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| ROS | Reactive oxygen species |
| PPAR-γ | Peroxisome proliferator-activated receptor gamma |
| Nrf2 | Nuclear factor erythroid 2–related factor 2 |
| IL-10 | Interleukin-10 |
| TNF-α | Tumor Necrosis Factor-alpha |
| CRP | C-Reactive Protein |
| LPS | Lipopolysacch < aride |
| ARDS | Acute respiratory distress syndrome |
| OGTT | Oral glucose tolerance test |
| ELISA | Enzyme-linked immunosorbent assay |
| LANCL2 | LanC-like protein 2 |
| GLUT4 | Glucose transporter type 4 |
| AMPK | AMP-activated protein kinase |
| PGC-1α | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| IDO | Indoleamine 2,3-dioxygenase |
| SD | Standard deviation |
| COPD | Chronic obstructive pulmonary disease |
References
- Slagter, S.N.; Vliet-Ostaptchouk, J.V.V.; Vonk, J.M.; Boezen, H.M.; Dullaart, R.P.F.; Kobold, A.C.M.; Feskens, E.J.; Beek, A.P.V.; Klauw, M.M.V.D.; Wolffenbuttel, B.H.R. Associations between smoking, components of metabolic syndrome and lipoprotein particle size. BMC Med. 2013, 11, 195. [Google Scholar] [CrossRef]
- Leone, A.; Landini, L.; Leone, A. What is Tobacco Smoke? Sociocultural Dimensions of the Association with Cardiovascular Risk. Curr. Pharm. Des. 2010, 16, 2510–2517. [Google Scholar] [CrossRef]
- Lin, C.C.; Li, C.I.; Liu, C.S.; Lin, C.H.; Yang, S.Y.; Li, T.C. Relationship between tobacco smoking and metabolic syndrome: A Mendelian randomization analysis. BMC Endocr. Disord. 2025, 25, 87. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, H.J.; Min, K.; Lee, H.; Lee, S.H.; Kim, S.; Kim, J.S.; Oh, B. The relationship between smoking cigarettes and metabolic syndrome: A cross-sectional study with non-single residents of Seoul under 40 years old. PLoS ONE 2021, 16, e0256257. [Google Scholar] [CrossRef]
- Filozof, C.; Fernández Pinilla, M.C.; Fernández-Cruz, A. Smoking cessation and weight gain. Obes. Rev. 2004, 5, 95–103. [Google Scholar] [CrossRef]
- Woodward, M.; Rumley, A.; Lowe, G.D.O.; Tunstall-Pedoe, H. C-reactive protein: Associations with haematological variables, cardiovascular risk factors and prevalent cardiovascular disease. Br. J. Haematol. 2003, 122, 135–141. [Google Scholar] [CrossRef]
- Nanda, R.; Patel, S.; Ghosh, A.; Asha, K.S.; Mohapatra, E. A study of apolipoprotein A1(ApoA1) and interleukin-10(IL-10) in diabetes with foot ulcers. BioMedicine 2022, 12, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, E.; Oberholzer, H.M.; Van Der Spuy, W.J.; Meiring, J.H. Smoking and coagulation: The sticky fibrin phenomenon. Ultrastruct. Pathol. 2010, 34, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Leone, A. Smoking, Haemostatic Factors, and Cardiovascular Risk. Curr. Pharm. Des. 2007, 13, 1661–1667. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, G.J.; Bressan, R.A.; Song, C.P.; Zhu, J.K.; Zhao, Y. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef]
- Nambara, E.; Marion-Poll, A. Abscisic acid biosynthesis and catabolism. Annu. Rev. Plant Biol. 2005, 56, 165–185. [Google Scholar] [CrossRef]
- Hoang, Q.T.M.; Nguyen, V.K.; Oberacher, H.; Fuchs, D.; Hernandez-Vargas, E.A.; Borucki, K.; Waldburg, N.; Wippermann, J.; Schreiber, J.; Bruder, D.; et al. Serum Concentration of the Phytohormone Abscisic Acid Is Associated with Immune-Regulatory Mediators and Is a Potential Biomarker of Disease Severity in Chronic Obstructive Pulmonary Disease. Front. Med. 2021, 8, 676058. [Google Scholar] [CrossRef] [PubMed]
- Magnone, M.; Sturla, L.; Guida, L.; Spinelli, S.; Begani, G.; Bruzzone, S.; Fresia, C.; Zocchi, E. Abscisic acid: A conserved hormone in plants and humans and a promising aid to combat prediabetes and the metabolic syndrome. Nutrients 2020, 12, 1724. [Google Scholar] [CrossRef] [PubMed]
- Schiano, E.; Maisto, M.; Piccolo, V.; Novellino, E.; Annunziata, G.; Ciampaglia, R.; Montesano, C.; Croce, M.; Caruso, G.; Iannuzzo, F.; et al. Beneficial Contribution to Glucose Homeostasis by an Agro-Food Waste Product Rich in Abscisic Acid: Results from a Randomized Controlled Trial. Foods 2022, 11, 2637. [Google Scholar] [CrossRef]
- Sturla, L.; Fresia, C.; Guida, L.; Bruzzone, S.; Scarfi, S.; Usai, C.; Fruscione, F.; Magnone, M.; Millo, E.; Basile, G.; et al. LANCL2 is necessary for abscisic acid binding and signaling in human granulocytes and in rat insulinoma cells. J. Biol. Chem. 2009, 284, 28045–28057. [Google Scholar] [CrossRef]
- Schiano, E.; Guerra, F.; Abate, F.; Piccinocchi, G.; Tenore, G.C.; Novellino, E. Exploring the Role of AbaComplex in Managing Dysglycemia: Insights from a Randomized, Three-Arm, Placebo-Controlled Trial. Diabetology 2025, 6, 14. [Google Scholar] [CrossRef]
- Bassaganya-Riera, J.; Guri, A.J.; Lu, P.; Climent, M.; Carbo, A.; Sobral, B.W.; Horne, W.T.; Lewis, S.N.; Bevan, D.R.; Hontecillas, R. Abscisic acid regulates inflammation via ligand-binding domain-independent activation of peroxisome proliferator-activated receptor γ. J. Biol. Chem. 2011, 286, 2504–2516. [Google Scholar] [CrossRef]
- Magnone, M.; Ameri, P.; Salis, A.; Andraghetti, G.; Emionite, L.; Murialdo, G.; De Flora, A.; Zocchi, E. Microgram amounts of abscisic acid in fruit extracts improve glucose tolerance and reduce insulinemia in rats and in humans. FASEB J. 2015, 29, 4783–4793. [Google Scholar] [CrossRef]
- Zocchi, E.; Hontecillas, R.; Leber, A.; Einerhand, A.; Carbo, A.; Bruzzone, S.; Tubau-Juni, N.; Philipson, N.; Zoccoli-Rodriguez, V.; Sturla, L.; et al. Abscisic Acid: A Novel Nutraceutical for Glycemic Control. Front. Nutr. 2017, 4, 24. [Google Scholar] [CrossRef]
- Sagliocchi, S.; Schiano, E.; Acampora, L.; Iannuzzo, F.; Cicatiello, A.G.; Miro, C.; Nappi, A.; Restolfer, F.; Stornaiuolo, M.; Zarrilli, S.; et al. AbaComplex Enhances Mitochondrial Biogenesis and Adipose Tissue Browning: Implications for Obesity and Glucose Regulation. Foods 2025, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- Bruzzone, S.; Bodrato, N.; Usai, C.; Guida, L.; Moreschi, I.; Nano, R.; Antonioli, B.; Fruscione, F.; Magnone, M.; Scarfì, S.; et al. Abscisic acid is an endogenous stimulator of insulin release from human pancreatic islets with cyclic ADP ribose as second messenger. J. Biol. Chem. 2008, 283, 32188–32197. [Google Scholar] [CrossRef]
- Magnone, M.; Emionite, L.; Guida, L.; Vigliarolo, T.; Sturla, L.; Spinelli, S.; Buschiazzo, A.; Marini, C.; Sambuceti, G.; De Flora, A.; et al. Insulin-independent stimulation of skeletal muscle glucose uptake by low-dose abscisic acid via AMPK activation. Sci. Rep. 2020, 10, 1454. [Google Scholar] [CrossRef]
- Spinelli, S.; Magnone, M.; Guida, L.; Sturla, L.; Zocchi, E. The ABA/LANCL Hormone/Receptor System in the Control of Glycemia, of Cardiomyocyte Energy Metabolism, and in Neuroprotection: A New Ally in the Treatment of Diabetes Mellitus? Int. J. Mol. Sci. 2023, 24, 1199. [Google Scholar] [CrossRef]
- Sturla, L.; Mannino, E.; Scarfì, S.; Bruzzone, S.; Magnone, M.; Sociali, G.; Booz, V.; Guida, L.; Vigliarolo, T.; Fresia, C.; et al. Abscisic acid enhances glucose disposal and induces brown fat activity in adipocytes in vitro and in vivo. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Schiano, E.; Novellino, E.; Gámez Fernández, M.M.; Tiekou Lorinczova, H.; Tenore, G.C.; Iannuzzo, F.; Patel, V.B.; Somavarapu, S.; Zariwala, M.G. Antioxidant and Antidiabetic Properties of a Thinned-Nectarine-Based Nanoformulation in a Pancreatic β-Cell Line. Antioxidants 2024, 13, 63. [Google Scholar] [CrossRef] [PubMed]
- Schiano, E.; Piccolo, V.; Novellino, E.; Maisto, M.; Iannuzzo, F.; Summa, V.; Tenore, G.C. Thinned Nectarines, an Agro-Food Waste with Antidiabetic Potential: HPLC-HESI-MS/MS Phenolic Characterization and In Vitro Evaluation of Their Beneficial Activities. Foods 2022, 11, 1010. [Google Scholar] [CrossRef]
- Vigliarolo, T.; Zocchi, E.; Fresia, C.; Booz, V.; Guida, L. Abscisic acid influx into human nucleated cells occurs through the anion exchanger AE2. Int. J. Biochem. Cell Biol. 2016, 75, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Hontecillas, R.; Roberts, P.C.; Carbo, A.; Vives, C.; Horne, W.T.; Genis, S.; Velayudhan, B.; Bassaganya-Riera, J. Dietary abscisic acid ameliorates influenza-virus-associated disease and pulmonary immunopathology through a PPARγ-dependent mechanism. J. Nutr. Biochem. 2013, 24, 1019–1027. [Google Scholar] [CrossRef]
- Wang, L.; Zou, H.; Xiao, X.; Wu, H.; Zhu, Y.; Li, J.; Liu, X.; Shen, Q. Abscisic acid inhibited reactive oxygen species-mediated endoplasmic reticulum stress by regulating the PPAR-γ signaling pathway in ARDS mice. Phytother. Res. 2021, 35, 7027–7038. [Google Scholar] [CrossRef]
- Zhao, C.C.; Xu, J.; Xie, Q.M.; Zhang, H.Y.; Fei, G.H.; Wu, H.M. Abscisic acid suppresses the activation of NLRP3 inflammasome and oxidative stress in murine allergic airway inflammation. Phyther. Res. 2021, 35, 3298–3309. [Google Scholar] [CrossRef]
- Antony, P.; Vijayan, R. Bioactive peptides as potential nutraceuticals for diabetes therapy: A comprehensive review. Int. J. Mol. Sci. 2021, 22, 9059. [Google Scholar] [CrossRef]
- Fernandes, I.; Oliveira, J.; Pinho, A.; Carvalho, E. The Role of Nutraceutical Containing Polyphenols in Diabetes Prevention. Metabolites 2022, 12, 184. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2025. Diabetes Care 2025, 48 Suppl. 1, S27–S49. [Google Scholar] [CrossRef]
- Schiano, E.; Neri, I.; Maisto, M.; Novellino, E.; Iannuzzo, F.; Piccolo, V.; Summa, V.; Grumetto, L.; Tenore, G.C. Validation of an LC-MS/MS Method for the Determination of Abscisic Acid Concentration in a Real-World Setting. Foods 2023, 12, 1077. [Google Scholar] [CrossRef]
- Spinelli, S.; Humma, Z.; Magnone, M.; Zocchi, E.; Sturla, L. Role of Abscisic Acid in the Whole-Body Regulation of Glucose Uptake and Metabolism. Nutrients 2025, 17, 13. [Google Scholar] [CrossRef]
- Agarwal, R. Smoking, oxidative stress and inflammation: Impact on resting energy expenditure in diabetic nephropathy. BMC Nephrol. 2005, 6, 13. [Google Scholar] [CrossRef]
- Kopp, W. Pathogenesis of (smoking-related) non-communicable diseases—Evidence for a common underlying pathophysiological pattern. Front. Physiol. 2022, 13, 1037750. [Google Scholar] [CrossRef]
- Addissouky, T.A.; El Sayed, I.E.T.; Ali, M.M.A.; Wang, Y.; El Baz, A.; Elarabany, N.; Khalil, A.A. Oxidative stress and inflammation: Elucidating mechanisms of smoking-attributable pathology for therapeutic targeting. Bull. Natl. Res. Cent. 2024, 48, 16. [Google Scholar] [CrossRef]
- Seo, Y.S.; Park, J.M.; Kim, J.H.; Lee, M.Y. Cigarette Smoke-Induced Reactive Oxygen Species Formation: A Concise Review. Antioxidants 2023, 12, 1732. [Google Scholar] [CrossRef] [PubMed]
- Elisia, I.; Lam, V.; Cho, B.; Hay, M.; Li, M.Y.; Yeung, M.; Bu, L.; Jia, W.; Norton, N.; Lam, S.; et al. The effect of smoking on chronic inflammation, immune function and blood cell composition. Sci. Rep. 2020, 10, 19480. [Google Scholar] [CrossRef]
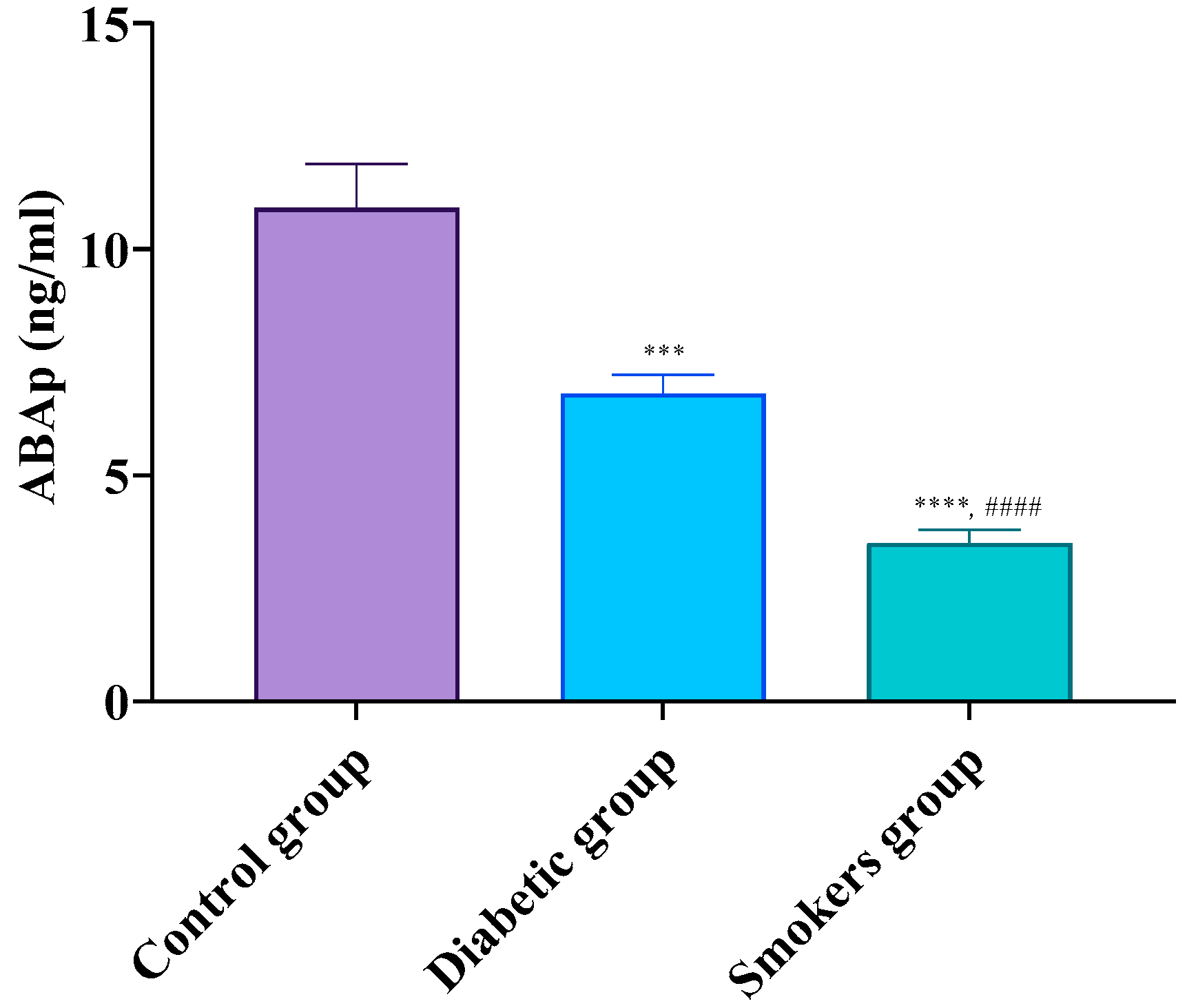
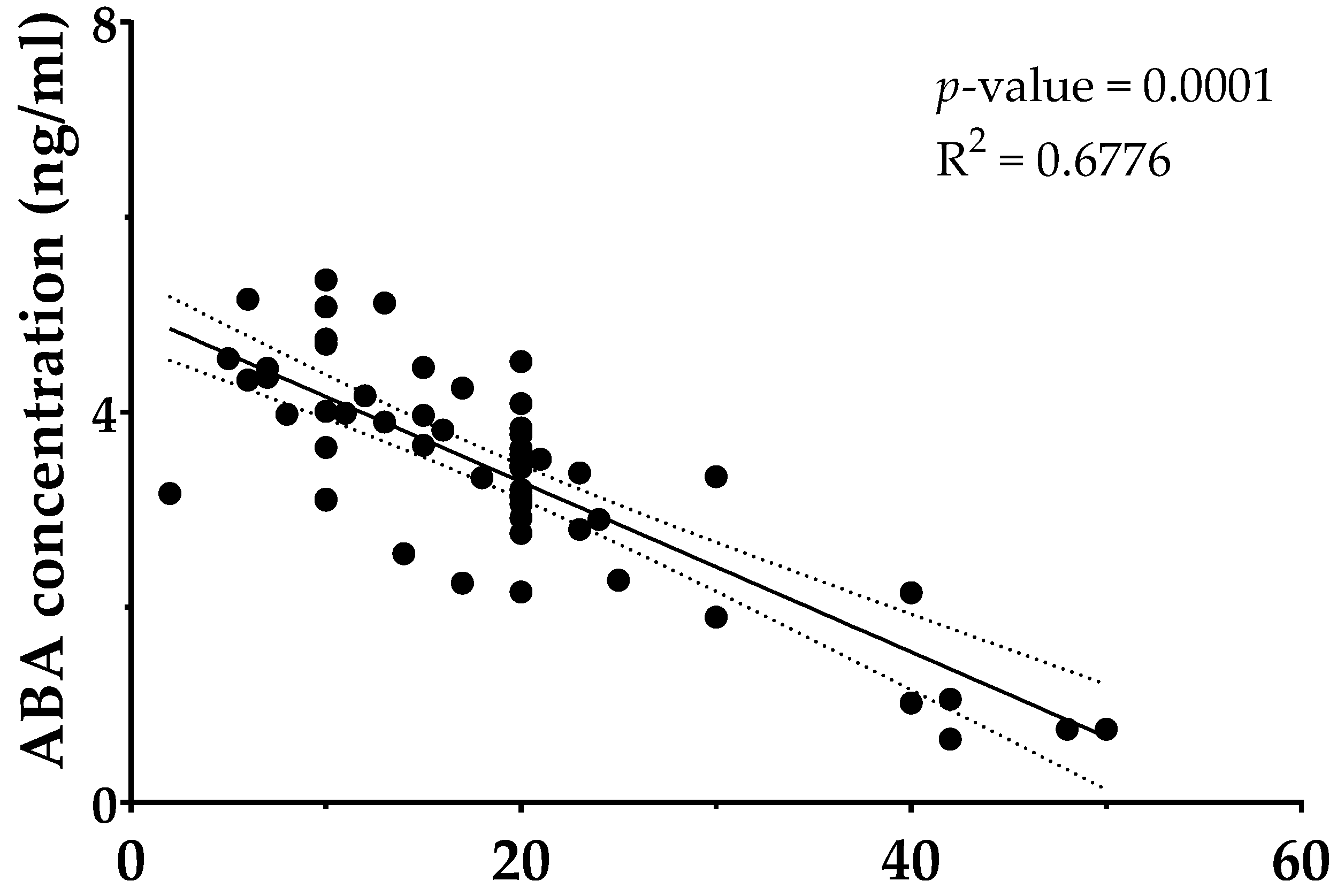
| Parameters | Control Group | Diabetic Group | Smokers Group |
|---|---|---|---|
| M/F | 33/19 | 60/41 | 36/20 |
| Age (years) | 61.6 ± 12.3 | 67.2 ± 10.7 | 63.5 ± 18.3 |
| BMI (kg/m2) | 25.1 ± 8.2 | 29.4 ± 7.4 | 26.7 ± 9.6 |
| Diabetes duration (years) | N/A | 10.2 ± 6.1 | N/A |
| FPG (mg/dL) | 84.2 ± 11.1 | 127.3 ± 50.5 **** | 93.3 ± 14.7 #### |
| HbA1c (%) | 5.0 ± 0.3 | 6.7 ± 1.6 | 5.0 ± 0.2 |
| Total cholesterol (mg/dL) | 180.4 ± 25.5 | 151.7 ± 39.9 | 164.3 ± 30.3 |
| HDL-cholesterol (mg/dL) | 44.0 ± 5.4 | 35.9 ± 7.3 | 43.5 ± 6.7 |
| LDL-cholesterol (mg/dL) | 83.7 ± 29.6 | 106.5 ± 20.2 * | 93.5 ± 22.5 |
| Triglyceride (mg/dL) | 92.6 ± 24.1 | 167.4 ± 18.1 **** | 106.0 ± 17.4 #### |
| ALT (UI/L) | 27.6 ± 11.6 | 25.0 ± 11.6 | 26.2 ± 10.7 |
| AST (UI/L) | 23.4 ± 7.5 | 19.0 ± 7.5 | 22.1 ± 9.4 |
| eGFR | 89.2 ± 15.3 | 78.6 ± 18.7 | 85.1 ± 14.9 |
| Uric acid (mg/dL) | 4.9 ± 0.7 | 4.5 ± 1.5 | 4.8 ± 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abate, F.; Schiano, E.; Guerra, F.; Piccinocchi, G.; Tenore, G.C.; Novellino, E. Abscisic Acid as a Marker of Metabolic Imbalance: Serum Levels from Diabetic and Smoking Subjects. Diabetology 2025, 6, 93. https://doi.org/10.3390/diabetology6090093
Abate F, Schiano E, Guerra F, Piccinocchi G, Tenore GC, Novellino E. Abscisic Acid as a Marker of Metabolic Imbalance: Serum Levels from Diabetic and Smoking Subjects. Diabetology. 2025; 6(9):93. https://doi.org/10.3390/diabetology6090093
Chicago/Turabian StyleAbate, Federico, Elisabetta Schiano, Fabrizia Guerra, Gaetano Piccinocchi, Gian Carlo Tenore, and Ettore Novellino. 2025. "Abscisic Acid as a Marker of Metabolic Imbalance: Serum Levels from Diabetic and Smoking Subjects" Diabetology 6, no. 9: 93. https://doi.org/10.3390/diabetology6090093
APA StyleAbate, F., Schiano, E., Guerra, F., Piccinocchi, G., Tenore, G. C., & Novellino, E. (2025). Abscisic Acid as a Marker of Metabolic Imbalance: Serum Levels from Diabetic and Smoking Subjects. Diabetology, 6(9), 93. https://doi.org/10.3390/diabetology6090093








