Recursive Interplay of Family and Biological Dynamics: Adults with Type 1 Diabetes Mellitus Under the Spotlight
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview
2.2. Participants
2.3. Sociodemographic, Cognitive and Clinical Features Characteristics
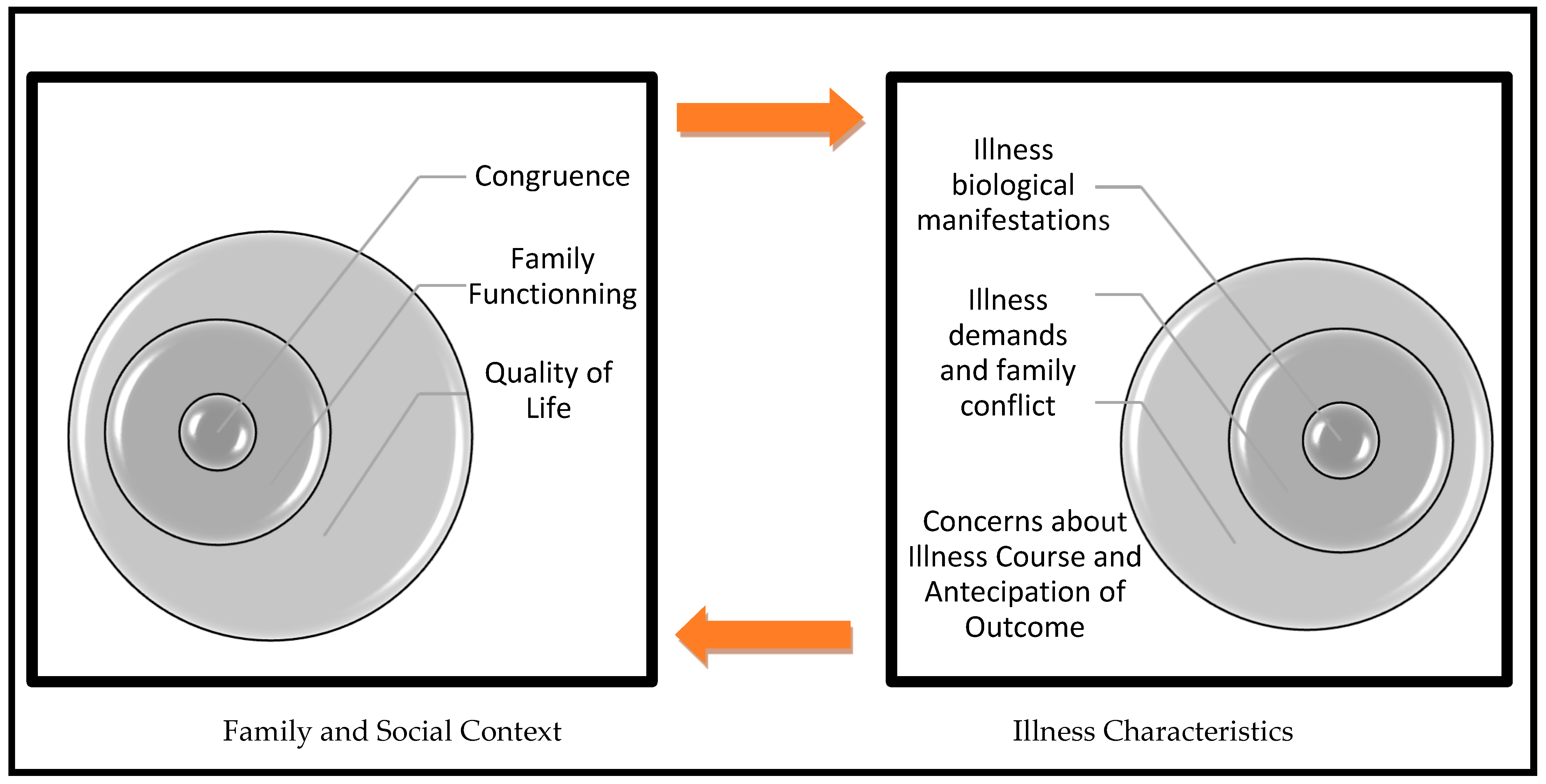
2.4. From Family to Diabetes Management
2.4.1. Individual Level as a Whole
2.4.2. Intrafamily Level
2.4.3. Extrafamily Level
2.5. From Diabetes Demands to Family Conflict
- The question, “How does diabetes management contribute to family conflict?”
- A list of Sources of conflict/support between the patient and the family due to diabetes, such as physical exercise, food restrictions, mealtimes, glycemic results and medical advice.
- Patients’ perception about their disease self-management (physical exercise, food, glycemic control, smoking habits), critical problems (food choice, future complications, lack of social support, hypoglycemic episodes, constant efforts to deal with disease) and Eating Behavior, assessed through Portuguese validation of Dutch Eating Behavior Questionnaire, DEBQ [72,73]. It is a 33-item instrument which evaluates three types of eating styles such as restrained (avoid eating more than initially defined), external (eating motivated by external factors such as the food’s good smell and appearance) and emotional (eating in response to emotions).
2.6. Data Analysis
3. Results
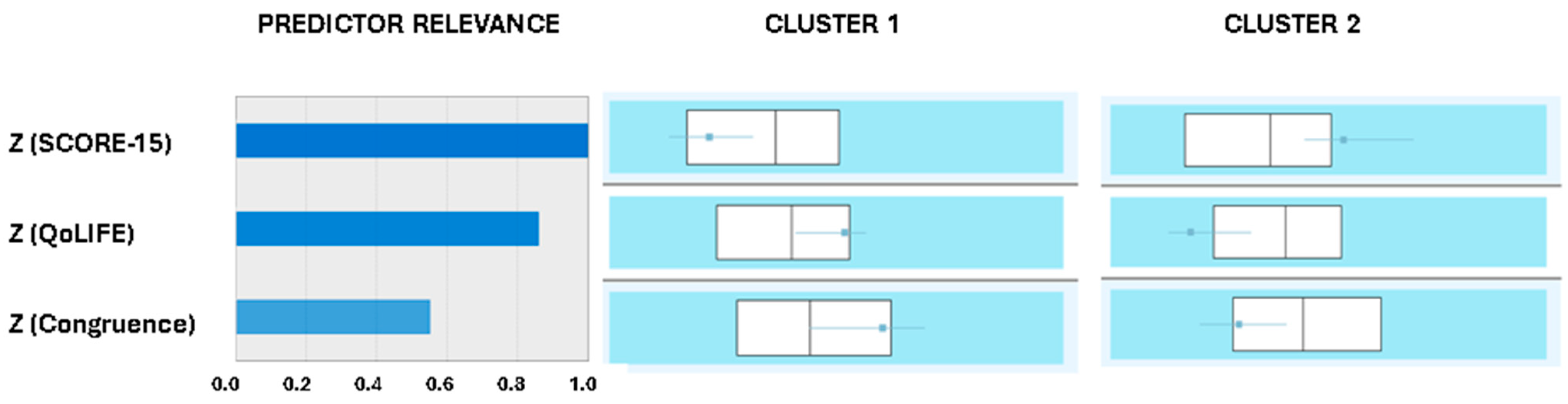
3.1. Two Cluster Solution and Metabolic Control Bipartition
3.2. Family System. Implications Diabetes Management
3.3. From the Demands of Diabetes to Family Conflict
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maturana, H.; Varela, F. A Árvore do Conhecimento: [The Tree of Knowledge] Jonas Pereira dos Santos. Workshopsy: São Paulo, 2015. Available online: https://pt.scribd.com/document/56555504/Arvore-Do-Conhecimento-Maturana-e-Varela (accessed on 6 October 2021).
- Young-Hyman, D.; De Groot, M.; Hill-Brigg, F.; Gonzalez, J.; Hood, K.; Peyrot, M. Psychosocial care for people with diabetes: A position statement of American Diabetes Association. Diabetes Care 2016, 39, 2126–2140. [Google Scholar] [CrossRef]
- American Diabetes Association. Lifestyle management: Standards of medical care in diabetes. Diabetes Care 2019, 42, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Rolland, J. Chronic illness and the life cycle: A conceptual framework. Fam. Process 1987, 26, 203–221. [Google Scholar] [CrossRef] [PubMed]
- Rolland, J. Families, Illness, and Disability: An Integrative Treatment Model; Basic Books: New York, NY, USA, 1994. [Google Scholar]
- Rolland, J. Mastering family challenges in serious illness and disability. In Normal Family Process; Guildford Press: New York, NY, USA, 2012; pp. 452–482. [Google Scholar]
- Engel, G. The Biopsychosocial model and the education of health professionals? Ann. N. Y. Acad. Sci. 1978, 310, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine. Health and Behavior: The Interplay of Biological, Behavioral, and Societal Influences; The National Academies Press: Washington, D.C., USA, 2001. [Google Scholar] [CrossRef]
- Morin, E. Introdução ao pensamento complexo. In Epistemologia e Sociedade, 4th ed.; Instituto Piaget: Almada, Portugal, 2003. [Google Scholar]
- White, M.; Epston, D. Narrative Means to Therapeutic Ends; W. W. Norton: New York, NY, USA, 1990. [Google Scholar]
- Rolland, J. A family psychosocial map with chronic conditions. In Helping Couples and Families Navigate Illness and Disability: An integrated Approach; Guilford Press: New York, NY, USA, 2018; pp. 3–18. [Google Scholar]
- Anderson, R.; Freelan, K.; Clouse, R.; Lustman, P. The prevalence of comorbid depression in adults with diabetes: A meta-analysis. Diabet. Care 2001, 24, 1069–1078. [Google Scholar] [CrossRef]
- Metsch, J.; Tillil, H.; Köbberling, J.; Sartory, G. On the relation among psychological distress, diabetes-related health behavior, and level of glycosylated hemoglobin in type 1 diabetes. Int. J. Behav. Med. 1995, 2, 104–117. [Google Scholar] [CrossRef]
- Taylor, M.; Frier, B.; Gold, A.; Deary, I. Psychosocial factors and diabetes-related outcomes following diagnosis of type 1 diabetes in adults: The Edinburgh prospective diabetes study. Diabet. Med. 2003, 20, 135–146. [Google Scholar] [CrossRef]
- Sultan, S.; Epel, E.; Sachon, C.; Vaillant, G.; Hartemann-Heurtier, A. A longitudinal study of coping, anxiety and glycemic control in adults with type 1 diabetes. Psychol. Health 2008, 23, 73–89. [Google Scholar] [CrossRef]
- Watts, S.; O’Hara, L.; Trigg, R. Living with type 1 diabetes: A by-person qualitative exploration. Psychol. Health 2010, 25, 491–506. [Google Scholar] [CrossRef]
- Strandberg, R.; Graue, M.; Wentzel-Larsen, T.; Peyrot, M.; Thordarson, H.; Rokne, B. Longitudinal relationship between diabetes-specific emotional distress and follow-up HbA1c in adults with type 1 diabetes mellitus. Diabet. Med. 2015, 32, 1304–1310. [Google Scholar] [CrossRef]
- De Groot, M.; Gold, S.; Wagner, S. Psychological conditions in adults with diabetes. Am. Psychol. 2016, 71, 552–562. [Google Scholar] [CrossRef]
- Hessler, D.; Fisher, L.; Polonsky, W.; Strycker, L.; Perters, A.; Blumer, I.; Bowyer, V. Diabetes distress is linked with worsening diabetes management over time in adults with Type 1 diabetes. Diabet. Med. 2017, 34, 1228–1234. [Google Scholar] [CrossRef]
- Anderbro, T.; Amsberg, S.; Moberg, E.; Gonder-Frederick, L.; Adamson, U.; Lins, E.; Johansson, U. A longitudinal study of fear of hypoglycaemia in adults with type 1 diabetes. Endocrinol. Diabet. Metab. 2018, 1, e00013. [Google Scholar] [CrossRef]
- Dunicheva, M.; Zagorovskaya, T.; Patrakeeva, E. The role of psychological features in management of patients with type 1 diabetes (case report). Georg. Med. News 2018, 277, 67–70. [Google Scholar] [PubMed]
- Rancourt, D.; Foster, N.; Bollepalli, B.; Fitterman, H.; Powers, M.; Clements, M.; Smith, L. Test of the modified dual pathway model of eating disorders in individuals with type 1 diabetes. Int. J. Eat. Disord. 2019, 52, 630–642. [Google Scholar] [CrossRef]
- Franks, M.; Sahin, Z.; Seidel, A.; Shields, C.; Oates, S.; Boushey, C. Table for two: Diabetes distress and diet-related interactions of married patients with diabetes and their spouses. Fam. Syst. Health 2012, 30, 154–165. [Google Scholar] [CrossRef]
- Ritholz, M.; Beste, M.; Edwards, S.; Beverly, E.; Atakov-Castillo, A.; Wolpert, H. Impact of continuous glucose monitoring on diabetes management and marital relationships of adults with type 1 diabetes and their spouses: A qualitative study. Diabet. Med. 2014, 31, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Lister, Z.; Wilson, C.; Fox, C.; Herring, R.; Simpson, C.; Smith, L. Partner expressed emotion and diabetes management among spouses living with Type 2 diabetes. Fam. Syst. Health 2016, 34, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Litchman, M.; Wawrzynski, S.; Allen, N.; Tracy, E.; Kelly, C.; Helgeson, V.S.; Berg, C.A. Yours, mine, and ours: A qualitative analysis of the impact of type 1 diabetes management in older adult married couples. Diabet. Spectr. 2019, 32, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Due-Christensen, M.; Willaing, I.; Ismail, K.; Forbes, A. Learning about type 1 diabetes and learning to live with it when diagnosed in adulthood: Two distinct but interrelated psychological processes of adaptation: A qualitative longitudinal study. Diabet. Med. 2018, 36, 742–752. [Google Scholar] [CrossRef]
- Latham, K. Chronic illness and families. Encycl. Fam. Stud. 2016, 1–5. [Google Scholar] [CrossRef]
- Peyrot, M.; Rubin, R.; Lauritzen, T.; Snoek, F.; Matthews, D.; Skovlund, S. Psychosocial problems and barriers to improved diabetes management: Results of the Cross-National Diabetes Attitudes, Wishes and Needs (DAWN) Study. Diabet. Med. A J. Br. Diabet. Assoc. 2005, 22, 1379–1385. [Google Scholar] [CrossRef]
- Ridge, K.; Treasure, J.; Forbes, A.; Thomas, S.; Ismail, K. Themes elicited during motivational interviewing to improve glycaemic control in adults with type 1 diabetes mellitus. Diabet. Med. 2011, 29, 148–152. [Google Scholar] [CrossRef]
- Spek, V.; Nefs, G.; Mommersteeg, P.; Speight, J.; Pouwer, F.; Denollet, J. Type D personality and social relations in adults with diabetes: Results from diabetes MILES—The Netherlands. Psychol. Health 2018, 33, 1456–1471. [Google Scholar] [CrossRef]
- Karlsen, B.; Bru, E. Coping styles among adults with type 1 and type 2 diabetes. Psychol. Health Med. 2002, 7, 245–259. [Google Scholar] [CrossRef]
- Smith, D.; Donnelly, P.; Howe, J.; Mumford, T.; Campbell, A.; Ruddock, A.; Wearden, A. A qualitative interview study of people living with well-controlled type 1 diabetes. Psychol. Health 2018, 33, 872–887. [Google Scholar] [CrossRef]
- McCarthy, M.; Grey, M. Type 1 diabetes self-management from emerging adulthood through older adulthood. Diabet. Care 2018, 41, 1608–1614. [Google Scholar] [CrossRef]
- Lister, Z.; Fox, C.; Wilson, C. Couples and diabetes: A 30-year narrative review of dyadic relation research. Contemp. Fam. Ther. 2013, 35, 613–638. [Google Scholar] [CrossRef]
- Wearden, A.; Ward, J.; Barrowclough, C.; Tarrier, N. Attributions for negative events in the partners of adults with type 1 diabetes: Associations with partners’ expressed emotion and marital adjustment. Br. J. Health Psychol. 2006, 11, 1–21. [Google Scholar] [CrossRef]
- Trief, P.; Sandberg, J.; Greenberg, R.; Graff, K.; Castronova, N.; Yoon, M.; Weinstock, R.S. Describing support: A qualitative study of couples living with diabetes. Fam. Syst. Health 2003, 21, 57–67. [Google Scholar] [CrossRef]
- Helgeson, V.; Berg, C.; Kelly, C.; Van Vleet, M.; Zajdel, M.; Tracy, E.; Litchman, M.L. Patient and partner illness appraisals and health among adults with type 1 diabetes. J. Behav. Med. 2019, 42, 480–492. [Google Scholar] [CrossRef]
- Hill, K.; Ward, P.; Gleadle, J. I kind of gave up on it after a while, became too hard, closed my eyes, didn’t want to know about it—adults with type 1 diabetes mellitus describe defeat in the context of low social support. Health Expect. 2018, 22, 254–261. [Google Scholar] [CrossRef]
- Roberson, P.; Fincham, F. Is relationship quality linked to diabetes risk and management? It depends on what you look at. Fam. Syst. Health 2018, 36, 315–326. [Google Scholar] [CrossRef]
- Schokker, M.; Links, T.; Bouma, J.; Keers, J.; Sanderman, R.; Wolffenbuttel, B. The role of overprotection by the partner in coping with diabetes: A moderated mediation model. Psychol. Health 2011, 26, 95–111. [Google Scholar] [CrossRef]
- Steinglass, P.; Horan, M. Families and chronic medical illness. In Chronic Disorders and the Families; The Hayworth Press: New York, NY, USA, 1988; pp. 127–142. [Google Scholar]
- Melo, A.; Alarcão, M. Beyond the family cycle: Understanding family development in the twenty-first century through complexity theories. Fam. Sci. 2014, 5, 55–59. [Google Scholar]
- Relvas, A.P. Instrumentos de Avaliação Familiar—Funcionamento e Intervenção [Family Assessment Instruments–Functioning and Intervention]; Imprensa da Universidade de Coimbra: Coimbra, Portugal, 2014; Volume I, pp. 9–20. Available online: https://eg-fr.uc.pt/bitstream/10316/41517/1/Avalia%C3%A7%C3%A3o%20Familiar.pdf (accessed on 4 April 2019).
- Walsh, F. Applying a family resilience framework in training, practice, and research: Mastering the art of the possible. Fam. Process 2016, 55, 616–632. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.; Stratton, P. The Score Family Assessment Questionnaire: A Decade of Progress. Fam. Process 2017, 56, 285–301. [Google Scholar] [CrossRef] [PubMed]
- White, P.; Smith, S.; O’Dowd, T. The role of the family in adult chronic Illness: A review of the literature on type 2 diabetes. Ir. J. Psychol. 2005, 26, 9–15. [Google Scholar] [CrossRef]
- Rintala, T.; Paavilainen, P.; Åstedt-Kurki, D. Everyday life of a family with diabetes as described by adults with type 1 diabetes. Eur. Diabetes Nurs. 2013, 10, 86–90. [Google Scholar] [CrossRef]
- López-Larrosa, S. Quality of life, treatment adherence, and locus of control: Multiple family groups for chronic medical illness. Fam. Process 2013, 52, 685–696. [Google Scholar] [CrossRef]
- Baig, A.; Benitez, A.; Quinn, M.; Burnet, D. Family interventions to improve diabetes outcomes for adults. Ann. N. Y. Acad. Sci. 2015, 1353, 89–112. [Google Scholar] [CrossRef]
- Dickinson, J.; Maryniuk, M. Building therapeutic relationships: Choosing words that put people first. Clin. Diabet. J. 2017, 35, 51–54. [Google Scholar] [CrossRef]
- Jacobson, A.; Hauser, S.; Cole, C.; Willett, J.; Wolfsdorf, J.; Dvorak, R.; Wolpert, H.; Herman, L.; de Groot, M. Social relationships among young adults with Insulin-dependent diabetes mellitus: Ten-year follow-up of an onset cohort. Diabet. Med. 1997, 14, 73–79. [Google Scholar] [CrossRef]
- Pinhas-Hamiel, O.; Tish, E.; Levek, N.; Bem-David, R.; Graf-Bar-El, C.; Yaron, M.; Boyko, V.; Lerner-Geva, L. Sexual lifestyle among young adults with type 1 diabetes. Diabet. Metab. Res. Rev. 2010, 33, e2837. [Google Scholar] [CrossRef]
- Palladino, D.; Helgeson, V.; Reynolds, K.; Becker, D.; Siminerio, L.; Escobar, O. Emerging adults with type 1 diabetes: A comparison to peers without diabetes. J. Pediatr. Psychol. 2013, 38, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Schabert, J.; Browne, J.; Mosely, K.; Speight, J. Social stigma in diabetes. Patient-Patient Centered Outcomes Res. 2013, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Helgeson, V.; Mascatelli, K.; Reynolds, K.; Becker, D.; Escobar, O.; Siminerio, L. Friendship and romantic relationships among emerging adults with and without type 1 diabetes. J. Pediatr. Psychol. 2015, 40, 359–372. [Google Scholar] [CrossRef]
- Robinson, C. Families living well with chronic illness: The healing process of moving on. Chronicity 2017, 27, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Martire, L.; Helgeson, V. Close relationships and management of chronic illness: Associations and interventions. Am. Psychol. 2017, 72, 601–612. [Google Scholar] [CrossRef]
- Wechsler, D. Escala de inteligência de Wechsler para adultos-Terceira Edição (WAIS-III) [WAIS-Wechsler Adult Intelligence Scale], 3rd ed.; Adaption, Validation and Normative Study for Portuguese Population; Cegoc: Lisbon, Portugal, 2008. [Google Scholar]
- Raven, J.; Raven, J.; Court, J. Matrizes Progressivas Coloridas de Raven, CPM-P; Cegoc: Lisboa, Portugal, 2009. [Google Scholar]
- Freitas, S.; Simões, M.; Alves, L.; Santana, I. Montreal Cognitive Assessment (MoCA): Normative study for the portuguese population. J. Clin. Exp. Neuropsychol. 2011, 33, 989–996. [Google Scholar] [CrossRef]
- Narciso, I.; Costa, M. Amores Satisfeitos, mas não perfeitos [Pleased but not perfect loves]. Cad. Consult. Psicológica. 1996, 12, 115–130. Available online: https://repositorio-aberto.up.pt/handle/10216/15550 (accessed on 4 April 2019).
- Lee, B. Development of a congruence scale based on the Satir model. Contemp. Fam. Ther. 2002, 24, 217–239. [Google Scholar] [CrossRef]
- Cunha, D.; Silva, J.; Relvas, A. Congruence scale (CS). In Instrumentos de Avaliação Familiar—Funcionamento e Intervenção [Family Assessment Instruments—Functioning and Intervention]; Relvas, A.P., Major, S., Eds.; Imprensa da Universidade de Coimbra: Coimbra, Portugal, 2014; Volume I, pp. 97–118. Available online: https://eg-fr.uc.pt/bitstream/10316/41517/1/Avalia%C3%A7%C3%A3o%20Familiar.pdf (accessed on 4 April 2019).
- Wretman, C. Saving Satir: Contemporary perspectives on the change process model. Soc. Work 2015, 61, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Walsh, F. Spiritual diversity: Multifaith perspectives in family therapy. Fam. Process 2010, 49, 330–348. [Google Scholar] [CrossRef]
- Stratton, P.; Bland, J.; Janes, E.; Lask, J. Developing an indicator of family function and a practicable outcome measure for systemic family and couple therapy: The SCORE. J. Fam. Ther. 2010, 32, 232–258. [Google Scholar] [CrossRef]
- Vilaça, M.; Silva, J.; Relvas, A. Systemic clinical outcome routine evaluation (SCORE-15). In Instrumentos de Avaliação Familiar—Funcionamento e Intervenção [Family Assessment Instruments—Functioning and Intervention]; Relvas, A.P., Major, S., Eds.; Imprensa da Universidade de Coimbra: Coimbra, Portugal, 2014; Volume I, pp. 23–41. Available online: https://eg-fr.uc.pt/bitstream/10316/41517/1/Avalia%C3%A7%C3%A3o%20Familiar.pdf (accessed on 4 April 2019).
- Simões, J. Qualidade de Vida. Estudo de Validação Para a População Portuguesa. [Quality of Life: Validation Study for Portuguese Population] (No Published Thesis Master). Faculty of Psychology and Educational Sciences: University of Coimbra. 2008. Available online: https://estudogeral.uc.pt/bitstream/10316/17369/3/Joana%20Sim%c3%b5es.pdf (accessed on 19 January 2019).
- Paddison, C. Family support and conflict among adults with type 2 diabetes. Eur. Diabetes Nurs. 2010, 7, 29–33. [Google Scholar] [CrossRef]
- Lewin, A.; Geffken, G.; Heidgerken, A.; Duke, D.; Novoa, W.; Williams, L.; Storch, E. The diabetes family behavior checklist: A psychometric evaluation. J. Clin. Psychol. Med. Settings 2005, 12, 315–322. [Google Scholar] [CrossRef]
- Van Strien, T.; Frijters, J.; Bergers, G.; Defares, P. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. Int. J. Eat. Disord. 1986, 5, 295–315. [Google Scholar] [CrossRef]
- Viana, V.; Sinde, S. Estilo alimentar: Adaptação e validação do questionário holandês do comportamento [Eating style: Adaptation and validation of the Dutch Eating Behavior Questionnaire]. Psicol. Teor. Investig. E Prática. 2003, 8, 59–71. Available online: https://www.researchgate.net/publication/236649218_ESTILO_ALIMENTAR_Adaptacao_e_validacao_do_Questionario_Holandes_do_Comportamento_Alimentar (accessed on 4 April 2019).
- Ghasemi, A.; Zahediasl, S. Normality tests for statistical analysis: A guide for non-statisticians. Int. J. Endocrinol. Metab. 2012, 10, 486–489. [Google Scholar] [CrossRef]
- Kos, A.; Psenicka, C. Measuring cluster similarity across methods. Psychol. Rep. 2000, 86, 858–862. [Google Scholar] [CrossRef]
- Maroco, J. Análise Estatística com Utilização do SPSS [Statistical Analysis Using SPSS], 3rd ed.; Edições Sílabo: Lisboa, Portugal, 2007. [Google Scholar]
- Cohen, J. Statistical Power Analysis. Curr. Dir. Psychol. Sci. 1992, 1, 98–101. [Google Scholar] [CrossRef]
- Fisher, L. Research on the family and chronic disease among adults: Major trends and directions. Fam. Syst. Health 2006, 24, 373–380. [Google Scholar] [CrossRef]
- Indelicato, L.; Calvo, V.; Dauriz, M.; Negri, A.; Negri, C.; Trombetta, M.; Bonora, E. Depressive symptoms and glycaemic control in adults with type 1 diabetes: An exploratory study on the role of family functioning. Acta Diabetol. 2020, 57, 23–30. [Google Scholar] [CrossRef]
- Urano, F. Wolfram Syndrome: Diagnosis, Management, and Treatment. Curr. Diabetes Rep. 2016, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Broadley, M.; Bishop, T.M.; Andrew, B. The relationship between attentional bias to food and disordered eating in females with type 1 diabetes. Appetite 2019, 140, 269–276. [Google Scholar] [CrossRef]
- Schafer, L.; McCaul, K.; Glasgow, R. Supportive and no supportive family behaviors: Relationships to adherence and metabolic Control in persons with type I diabetes. Diabet. Care 1986, 9, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, A.; Samson, J.; Weinger, K.; Ryan, C. Diabetes, the brain, and behavior: Is there a biological mechanism underlying the association between diabetes and depression? Int. Rev. Neurobiol. 2002, 51, 455–479. [Google Scholar] [CrossRef]
- Fonseca, G.; Cunha, D.; Crespo, C.; Relvas, A. Families in the context of macroeconomic crises: A systematic review. J. Fam. Psychol. 2016, 30, 687–697. [Google Scholar] [CrossRef]
- Pereira, M.; Pedras, S.; Machado, J. Family variables as moderators between beliefs towards medicines and adherence to self-care behaviors and medication in type 2 diabetes. Fam. Syst. Health 2014, 32, 198–206. [Google Scholar] [CrossRef]
- Hunter, C. Understanding diabetes and the role of psychology in its prevention and treatment. Am. Psychol. 2016, 71, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Jonhson, S. Increasing psychology’s role in health research and health care. Am. Psychol. 2013, 68, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Olson, D. FACES IV and the Circumplex Model: Validation study. J. Marital Fam. Ther. 2011, 37, 64–80. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, E.; Carr, A. Systematic review of self-report family assessment measures. Fam. Process 2016, 55, 16–30. [Google Scholar] [CrossRef]
- Jonhson, S.; Marrero, D. Innovations in healthcare delivery and policy: Implications for the role of the psychologist in preventing and treating diabetes. Am. Psychol. 2016, 71, 628–637. [Google Scholar] [CrossRef]
| Parameters | Group With Metabolic Control N = 49 | Group No Metabolic Control N = 42 | X2 | t | U | gl | p | d | |
|---|---|---|---|---|---|---|---|---|---|
| Sociodemographic data | |||||||||
| Gender | Male | 31 | 25 | 0.13 | 0.82 | 0.07 | |||
| Female | 18 | 17 | |||||||
| Age (in years) | 37.2 ± 9.4 | 36.2 ± 8.6 | 0.53 | 89 | 0.59 | −0.11 | |||
| Marital Status | Single | 22 | 24 | 1.37 | 1 | 0.24 | 0.07 | ||
| Couple | 27 | 18 | |||||||
| Household | Alone | 17 | 16 | 1.69 | 1 | 0.43 | 0.08 | ||
| Couple | 28 | 21 | |||||||
| With children | 3 | 5 | |||||||
| Income | Stable | 33 | 16 | 8.94 | 1 | 0.003 | 0.66 | ||
| Instable | 151 | 26 | |||||||
| Residence (Hospital distance) | Near | 20 | 16 | 2.97 | 2 | 0.23 | 0.36 | ||
| ≤1 h | 2 | 17 | |||||||
| >1 h | 16 | 9 | |||||||
| Area of Residence | Urban | 38 | 28 | 1.98 | 2 | 0.37 | 0.06 | ||
| Semi-urban | 8 | 8 | |||||||
| Rural | 3 | 6 | |||||||
| Educational level | ≤12 years | 17 | 27 | 7.93 | 1 | 0.005 | 0.61 | ||
| >12 years | 32 | 15 | |||||||
| DM data | |||||||||
| Disease onset | <18 ys | 24 | 24 | 0.61 | 1 | 0.38 | 0.16 | ||
| ≥18 ys | 25 | 18 | |||||||
| DM Duration | 17.5 ± 10.3 | 17.2 ± 9.5 | −0.16 | 89 | 0.87 | −0.03 | |||
| HbA1c (%) * | 7.2 ± 0.6 | 8.5 ± 1.2 | 6.32 | 89 | <0.001 | 0.07 | |||
| BMI | 24.9 ± 3.3 | 25.2 ± 3.8 | 989 | 0.75 | 0.06 | ||||
| Complications | Yes | 21 | 30 | 7.94 | 1 | 0.006 | 0.62 | ||
| No | 28 | 12 | |||||||
| Smoking status | Yes | 11 | 7 | 0.48 | 1 | 0.49 | 0.14 | ||
| No | 38 | 35 | |||||||
| Cognitive data | |||||||||
| Vocabulary | 32.33 ± 3.4 | 33.60 ± 2.8 | 807 | 0.075 | 0.03 | ||||
| Digit Memory | 14.7 ± 2.1 | 14.10 ± 1.9 | 1273 | 0.05 | 0.41 | ||||
| RPMT | 8.04 ± 0.9 | 8.05 ±1.0 | 981 | 0.69 | 0.08 | ||||
| Parameters | Group With MC (n = 49) | Group No MC (n = 42) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | 1st Q | 2nd Q | 3rd Q | M | SD | 1st Q | 2nd Q | 3rd Q | U | t | gl | p | d | |
| Score-15 | |||||||||||||||
| Family Strengths | 1.6 | 0.6 | 1.4 | 1.8 | 2.1 | 1.8 | 0.6 | 1.2 | 1.4 | 2.0 | 1141.5 | 0.002 | 0.33 | ||
| Family Difficulties | 1.6 | 0.6 | 1.8 | 2.6 | 2.8 | 2.4 | 0.7 | 1.2 | 1.6 | 2.0 | 411 | <0.001 | 1.22 | ||
| Family Communication | 1.8 | 0.6 | 2.2 | 2.8 | 3.2 | 2.68 | 0.7 | 1.4 | 1.8 | 2.1 | 42.3 | <0.001 | 1.16 | ||
| Congruence | |||||||||||||||
| Intra/Interpersonal | 48.5 | 7.3 | 45.5 | 50.0 | 53.5 | 42.8 | 8.3 | 37.7 | 42.0 | 48.0 | 1495.5 | <0.001 | −0.73 | ||
| Universal Congruence | 33.7 | 10.7 | 28.5 | 35.0 | 42.0 | 25.4 | 9.2 | 17.0 | 25.0 | 32.0 | 1504 | <0.001 | −0.83 | ||
| Quality of Life | |||||||||||||||
| Financial | 22.3 | 4.8 | 19.0 | 22.0 | 27.0 | 19.1 | 4.3 | 16.0 | 19.0 | 22.0 | 1430.5 | <0.001 | −0.72 | ||
| Time | 12.4 | 2.9 | 11.0 | 13.0 | 15.0 | 11.5 | 2.1 | 10.0 | 11.5 | 13.0 | −1.56 | 89 | 0.120 | −0.33 | |
| Neighborhood | 20.3 | 3.7 | 18.0 | 20.0 | 23.0 | 18.3 | 3.3 | 16.0 | 18.0 | 21.0 | −2.69 | 89 | 0.009 | −0.57 | |
| Home Conditions | 18.2 | 3.3 | 16.0 | 18.0 | 20.0 | 18.3 | 3.0 | 16.0 | 18.5 | 20.2 | 0.16 | 89 | 0.870 | 0.03 | |
| Mass Media | 9.2 | 2.1 | 8.0 | 9.0 | 10.0 | 9.2 | 2.5 | 7.0 | 9.0 | 11.0 | 0.03 | 89 | 0.978 | 0.01 | |
| Social/Health Relationships | 14.9 | 2.4 | 14.0 | 15.0 | 16.0 | 12.8 | 2.4 | 11.0 | 13.0 | 14.0 | 1553 | <0.001 | −0.86 | ||
| Job | 9.9 | 2.7 | 8.5 | 10.0 | 11.5 | 8.6 | 2.2 | 7.0 | 8.0 | 10.0 | 2.44 | 89 | 0.017 | −0.51 | |
| Religion | 6.3 | 1.9 | 6.0 | 6.0 | 8.0 | 5.1 | 2.3 | 3.0 | 5.0 | 7.0 | 1365 | 0.006 | −0.64 | ||
| Family/Marital | 8.2 | 1.7 | 8.0 | 8.0 | 10.0 | 6.9 | 1.9 | 6.0 | 7.0 | 8.0 | 1447.5 | 0.001 | −0.72 | ||
| Children | 6.9 | 2.1 | 5.0 | 7.0 | 9.0 | 7.0 | 2.1 | 5.0 | 7.5 | 8.2 | 1026 | 0.981 | 0.03 | ||
| Education | 7.4 | 1.4 | 7.0 | 8.0 | 8.0 | 6.4 | 1.4 | 5.0 | 6.0 | 8.0 | 1411.5 | 0.002 | −0.73 | ||
| Parameters | Binary Logistic Regression No Metabolic Control Category | |||
|---|---|---|---|---|
| B | Exp(B) | 95%IC | p | |
| Sociodemographic data | ||||
| Income | −1.22 | 0.29 | 0.12–0.74 | 0.009 |
| Level of education | −1.29 | 0.27 | 0.11–0.68 | 0.005 |
| Clinical data | ||||
| HbA1c Values | 1.73 | 5.62 | 2.59–12.24 | <0.001 |
| Family System | ||||
| SCORE-15 | 1.43 | 4.17 | 1.15–11.27 | 0.005 |
| Congruence | −0.05 | 0.96 | 0.92–0.99 | 0.01 |
| Eating Behavior | ||||
| Emotional Ingestion | 0.69 | 2.27 | 1.24–4.13 | 0.008 |
| Parameters | Group With Metabolic Control (N = 27) | Group No Metabolic Control (N = 18) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | 1st Q | 2nd Q | 3rd Q | M | SD | 1st Q | 2nd Q | 3rd Q | U | p | d | |
| Total Marital Functioning | 3.8 | 0.7 | 3.4 | 3.8 | 4.3 | 2.8 | 0.6 | 2.2 | 2.7 | 3.4 | 406 | <0.001 | 1.21 |
| Family Functioning | 4.7 | 1.1 | 3.8 | 4.7 | 6.0 | 3.6 | 1.0 | 2.5 | 3.3 | 4.5 | 376.5 | 0.002 | 1.29 |
| Free Time | 3.9 | 1.1 | 3.0 | 4.0 | 5.0 | 2.9 | 0.9 | 2.0 | 3.0 | 3.6 | 365.5 | 0.004 | 1.33 |
| Autonomy | 3.9 | 1.0 | 4.0 | 4.7 | 5.5 | 4.7 | 0.9 | 3.0 | 3.7 | 4.7 | 353 | 0.010 | 1.36 |
| Extra Fam Relationships | 3.7 | 0.9 | 4.3 | 4.7 | 6.0 | 4.9 | 0.8 | 3.0 | 3.6 | 4.1 | 408 | <0.001 | 1.21 |
| Communication & Conflict | 3.5 | 1.0 | 4.5 | 5.0 | 5.7 | 4.9 | 1.0 | 3.0 | 3.1 | 4.5 | 400 | <0.001 | 1.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jorge, H.; Correia, B.R.; Castelo-Branco, M.; Relvas, A.P. Recursive Interplay of Family and Biological Dynamics: Adults with Type 1 Diabetes Mellitus Under the Spotlight. Diabetology 2025, 6, 81. https://doi.org/10.3390/diabetology6080081
Jorge H, Correia BR, Castelo-Branco M, Relvas AP. Recursive Interplay of Family and Biological Dynamics: Adults with Type 1 Diabetes Mellitus Under the Spotlight. Diabetology. 2025; 6(8):81. https://doi.org/10.3390/diabetology6080081
Chicago/Turabian StyleJorge, Helena, Bárbara Regadas Correia, Miguel Castelo-Branco, and Ana Paula Relvas. 2025. "Recursive Interplay of Family and Biological Dynamics: Adults with Type 1 Diabetes Mellitus Under the Spotlight" Diabetology 6, no. 8: 81. https://doi.org/10.3390/diabetology6080081
APA StyleJorge, H., Correia, B. R., Castelo-Branco, M., & Relvas, A. P. (2025). Recursive Interplay of Family and Biological Dynamics: Adults with Type 1 Diabetes Mellitus Under the Spotlight. Diabetology, 6(8), 81. https://doi.org/10.3390/diabetology6080081







