Abstract
Background and Aim: Type 2 diabetes (T2D) continues to pose a significant public health challenge worldwide. Among therapeutic options, glucagon-like peptide-1 receptor agonists (GLP-1 RAs) have proven effective in optimizing glycemic control and improving cardiometabolic profiles. Semaglutide, now available in an oral formulation, represents a modern strategy to improve patient adherence while supporting glucose and weight regulation. This study primarily investigated the effects of oral semaglutide on key metabolic indicators and secondary endpoints included cardiovascular risk markers (blood pressure and lipid profile) and patient-reported quality of life (QoL). Study Design and Methods: A longitudinal, prospective observational study was conducted involving patients with T2D across two Italian healthcare facilities. Participants were assessed at baseline (T0) and at three subsequent intervals—6 months (T1), 12 months (T2), and 18 months (T3)—following the initiation of oral semaglutide use. Key Findings: Out of 116 participants enrolled, 97 had complete and analyzable data. Across the 18-month follow-up, significant improvements were observed in glycemic parameters, with a notable reduction in HbA1c levels (T0 vs. T3, p = 0.0028; p ≤ 0.05, statistically significant). Self-reported outcomes showed enhanced quality of life, especially in treatment satisfaction and perceived flexibility (T0 vs. T3, p < 0.001). Conclusions: Daily administration of 14 mg oral semaglutide in individuals with T2D resulted in substantial benefits in glycemic regulation, weight reduction, cardiovascular risk management, and overall patient satisfaction. These findings reinforce its potential role as a sustainable and effective option in long-term diabetes care from both a clinical and public health perspective.
1. Introduction
Type 2 diabetes (T2D) represents a growing challenge for public health systems globally, significantly impacting both life expectancy and quality of life (QoL) [1]. According to the World Health Organization (WHO), the global burden of diabetes has expanded dramatically, with prevalence rates increasing fourfold over the past thirty years [2,3]. Projections by the International Diabetes Federation (IDF) further highlight this concerning trend, estimating a rise in global diabetes prevalence from 10.5% in 2021 to 11.3% by 2030, and reaching 12.2% by 2040 [4,5]. In Italy, national statistics from the Italian National Institute of Statistics (ISTAT) indicate that, as of 2016, more than 3.2 million people (approximately 5.3% of the population) are living with diabetes. This figure rises significantly among older adults, with a prevalence of 16.5% in individuals over 65. Contributing lifestyle factors are also noteworthy: in the 45–64 age group, 28.9% of men and 32.8% of women were classified as both obese and physically inactive [6]. There is a well-established association between T2D and measures of adiposity, particularly body mass index (BMI) and waist circumference (WC). These indicators of central fat distribution are strong predictors of metabolic dysfunction and are closely correlated with the risk of developing T2D. As such, the control of weight and abdominal fat emerges as a key strategy in both the prevention and management of the disease and its related complications, from both clinical and public health perspectives [1,2,3,4,5]. There are different pharmacological approaches for T2D treatment, among which glucagon-like peptide-1 receptor agonists (GLP-1 RAs) stand out due to their remarkable efficacy in lowering glucose levels and addressing other cardiometabolic risk factors [7,8]. These medications, also referred to as incretin mimetics, function by boosting the GLP-1 hormone, providing a safer option with a reduced risk of hypoglycemia [9]. Current treatment guidelines for T2D recommend using GLP-1 receptor agonists (GLP-1 RAs) as either a primary or secondary therapy (following metformin) due to their positive effects on weight and their beneficial influence on heart and kidney function. GLP-1 RAs contribute to body weight (BW) loss, improve blood pressure (BP), and enhance lipid profiles (LP), while also demonstrating anti-inflammatory and anti-atherosclerotic properties. These characteristics help reduce cardiovascular and renal risks, in addition to managing blood glucose (BG) levels [10,11,12,13]. Therapeutic adherence and QoL are crucial factors in managing a chronic condition like T2D. Several studies have explored these aspects, alongside traditional clinical and metabolic outcomes, in relation to semaglutide in both subcutaneous and oral formulations [14,15,16,17]. Semaglutide is the first oral GLP-1 RA, co-formulated with sodium N-[8-(2-hydroxybenzoyl) amino caprylate] (SNAC), to enhance absorption and bioavailability by protecting it from enzymatic degradation. The once-daily oral formulation was developed to simplify treatment initiation and improve adherence, particularly among patients who are new to injections [18,19]. Research indicates that oral semaglutide may result in higher adherence rates, compared to injectable forms [20,21,22,23]. The PIONEER program, which included extensive phase 3 clinical trials, demonstrated that oral semaglutide is non-inferior to placebo in terms of cardiovascular safety and leads to significant improvements in blood glucose control, BW, BMI, and WC [15,17,24,25,26,27,28,29,30,31,32]. However, further real-world studies are necessary to validate clinical trial findings and assess the broader effects of oral semaglutide on BG, weight management, cardiovascular risks, and QoL across diverse patient populations [16,33,34,35,36,37].
Aims and Research Questions
This study was designed to primarily investigate the multifaceted effects of oral semaglutide in individuals living with T2D, with a particular focus on its potential to improve clinical and metabolic outcomes, as well as patient-reported QoL in routine care settings. The oral route of administration, being less invasive than injectables, may enhance QoL, which was assessed using integrated quantitative and qualitative tools not commonly employed in clinical studies [38,39] (Supplementary Files S2 and S3). In contrast to previous randomized controlled trials conducted in Japan [40], this prospective observational study was carried out in real-world clinical practice in Italy, providing valuable insights into the effectiveness and patient experience associated with oral semaglutide in this specific population and healthcare context, with a notable final follow-up period of 18 months. The study aimed to answer the following questions:
- -
- Does oral semaglutide lead to a significant reduction in clinical–metabolic outcomes?
- -
- Does the use of oral semaglutide improve self-reported QoL, treatment satisfaction, and perceived health status as measured by validated tools [38,39]?
2. Methods
2.1. Study Design
This prospective observational clinical study was conducted at two Italian clinical centers in the Marche Region: the Diabetology and Endocrinology Units of AST Fermo (Fermo) and AST Ancona (Jesi). The STROBE checklist was adopted for study reporting (Supplementary File S3) [41].
2.2. Ethical Considerations
All procedures complied with the ethical standards of the relevant institutional and national committees for human research, as well as the Declaration of Helsinki. The study protocol was approved by the CERM Regional Ethics Committee (protocol number 2022/123-8040) on 17 October 2024. Informed consent was obtained from all participants prior to their inclusion in the study.
2.3. Sample and Criteria
All patients who began treatment with oral semaglutide between July 2022 and October 2022 at the recruitment centers were included in the study and followed according to the study timeline.
2.3.1. Inclusion Criteria
Participants were eligible for inclusion if they had a confirmed diagnosis of T2D, were of either sex, and were aged 18 years or older. Eligibility also required a clinical indication for treatment intensification, as assessed by the referring clinic, and the provision of signed written informed consent.
2.3.2. Exclusion Criteria
Exclusion criteria included a known intolerance or contraindications to oral semaglutide, prior use of GLP-1 receptor agonists or sodium-glucose cotransporter 2 (SGLT2) inhibitors, and the presence or suspicion of malignant conditions such as cancer. Patients were also excluded if they were pregnant or breastfeeding, had experienced a recent acute illness (other than viral infections) within the past three months, or had renal failure, defined as an estimated glomerular filtration rate (eGFR) below 60 mL/min. Additional exclusion criteria were advanced liver disease, congestive heart failure classified as New York Heart Association (NYHA) class IV, proliferative diabetic retinopathy, gallstone disease, chronic or acute pancreatitis, or adherence to a ketogenic or very-low-carbohydrate diet.
2.4. Outcomes Analyzed
- -
- Primary outcomes analyzed: glycated hemoglobin (HbA1c, %), fasting plasma glucose (FPG, mg/dL), and body composition and anthropometric parameters, including BW (kg), BMI (kg/m2), body water (kg), fat mass (%), and muscle mass (%).
- -
- Secondary outcomes analyzed: systolic blood pressure (SBP, mmHg), diastolic blood pressure (DBP, mmHg), total cholesterol (TC, mg/dL), low-density lipoprotein cholesterol (LDL-C, mg/dL), high-density lipoprotein cholesterol (HDL-C, mg/dL), triglycerides (TG, mg/dL), and QoL adopting the Italian versions of the Short Form Health Survey (SF-36) [38] and the WHO-Diabetes Treatment Satisfaction Questionnaire (WHO-DTSQ) (original version Supplementary File S1 and S2) [39].
2.5. Timing and Tools
According to standard care [8], oral semaglutide was prescribed in a stepwise regimen, starting with 3 mg/day during the first month, 7 mg/day in the second month, and escalating to 14 mg/day for subsequent months, with follow-ups at 6 (T1), 12 (T2), and 18 (T3) months. In alignment with clinical practice, patients enrolled in the study received a tailored nutritional intervention with recommended macronutrient intake percentages: 45–60% carbohydrates, 10–20% proteins, and 25–30% fats, alongside a caloric intake of 25–30 kcal/kg (ideal weight). At baseline (T0), beyond the outcomes described above, the following data were collected for each patient: age, gender, and duration. Body composition was assessed using routine Bioelectrical Impedance Analysis (BIA) performed with a DC-430MA™ device (TANITA Europe BV, Amsterdam, The Netherlands). Finally, the QoL was evaluated using the validated Italian versions of the WHO-DTSQ and SF-36 questionnaires [38,39]. Data on clinical parameters, BIA results, and QoL scores were also collected at follow-up visits (T1, T2, and T3). The SF-36, one of the most widely used instruments for assessing health-related quality of life (HRQOL), consists of 36 items encompassing eight dimensions: physical functioning, role limitations due to physical health problems, bodily pain, general health perception, vitality, social functioning, role limitations due to emotional health problems, and mental health [38]. The SF-36 has been extensively validated in large population studies and across various clinical chronic care settings, demonstrating robust psychometric properties [42,43]. The WHO-DTSQ was specifically designed to evaluate satisfaction with diabetes management [39]. The survey consists of eight items designed to assess patient satisfaction and self-perceptions related to diabetes management over the previous 15 days. The items evaluate the following aspects: (1) overall satisfaction with the current treatment; (2) perceived frequency of hyperglycemia; (3) perceived frequency of hypoglycemia; (4) convenience and ease of the treatment; (5) flexibility of the treatment regimen; (6) satisfaction with diabetes knowledge; (7) likelihood of recommending the treatment to others; and (8) willingness to continue the current treatment. This comprehensive evaluation provides valuable insight into self-reported outcomes, complementing clinical parameters and aiding in the holistic assessment of treatment effectiveness and acceptability. It comprises eight items, six of which are summed into a single score ranging from 0 (very dissatisfied) to 36 (very satisfied), providing a reliable measure of patient satisfaction with their treatment.
2.6. Statistical Analysis
For the statistical analysis, the dataset was first imported into a Pandas DataFrame (https://pandas.pydata.org/pandas-docs/stable/reference/api/pandas.DataFrame.html accessed on 1 April 2025). To preserve the majority of the information without introducing bias, missing data were processed using the pairwise deletion methodology, which is widely recognized as less biased for data missing completely at random (MCAR) or missing at random (MAR) [44,45]. The dataset contained various health and clinical parameters measured at baseline (T0) and after 6 (T1), 12 (T2), and 18 months (T3) of treatment with oral semaglutide (14 mg/day). To select the appropriate statistical test, normality tests for each variable were performed using the Shapiro–Wilk test. The data distribution of the sample was found to be generally non-normal. Given this tendency, differences between the various endpoints (T0, T1, T2, T3) were analyzed using a repeated-measures ANOVA with the non-parametric Friedman test. Post-hoc pairwise comparisons were conducted using the Durbin–Conover test to identify significant differences between time points. For the analysis, the open-source statistical software Jamovi was used (https://www.jamovi.org/ accessed on 1 April 2025). For each pair of measurements, the following statistical parameters were computed: mean; median; standard deviation (SD), which measures the spread of the differences around the mean difference; standard error of the mean (SE Mean), indicating the SD of the sample mean difference, which estimates the precision of the mean difference; 95% confidence interval (CI), which defines the range where the true mean difference is likely to lie with 95% confidence; W statistic, representing the calculated value for the Shapiro–Wilk test; and p-value, indicating the probability of having a non-normal distribution.
3. Results
3.1. Sample and Baseline Characteristics of Participants
A total of 116 patients were enrolled, of which 97 achieved sufficient compliance for the quantitative and aggregated analysis of the data (Figure 1): Two participants did not provide consent, fourteen dropped out (eight due to mild gastrointestinal side effects such as nausea, vomiting, and diarrhea, and six due to other minor side effects including headache, fatigue, and appetite changes), and three were excluded for non-compliance with study procedures. At recruitment, the median age of the sample was 64 years [interquartile range (IQR): 57–69]. The median duration since diabetes diagnosis was 5 years (IQR: 3–9). The mean weight was 94.5 ± 15.5 kg, and the average height was 1.77 ± 0.09 m. The median BMI was 34.30 kg/m2 (IQR: 29.35–38.18). For glycemic parameters, the median FPB was 146 mg/dL (IQR: 122–173), and the median HbA1c% was 7.69% (IQR: 7–8.46). Regarding lipid profile, the mean HDL-C levels were 45.07 ± 10.47 mg/dL, the mean LDL-C levels were 100.32 ± 34.52 mg/dL, and the median TGs were 148 mg/dL (IQR: 122–190). Detailed baseline characteristics are summarized in Table 1.
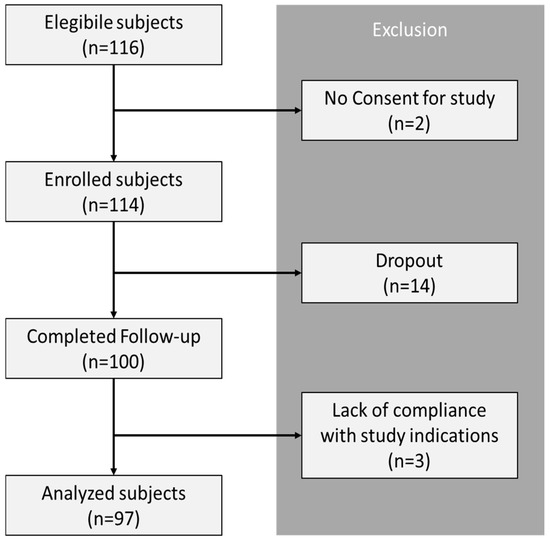
Figure 1.
Study flow chart.

Table 1.
Baseline characteristics.
3.2. Time Evolution and Variable Correlations
Metabolic results demonstrated significant changes in biochemical parameters relevant to clinical outcomes, specifically regarding T0 vs. T1, T0 vs. T2, T0 vs. T3, T1 vs. T2, and T1 vs. T3 for most variables analyzed. Notably, almost all variables showed statistically significant improvements over the longest comparison period (T0 vs. T3), with the exception of HDL-C, TG, SBP, and DBP, which did not exhibit significant changes across any of the periods considered. Interestingly, HbA1c% presented a statistically significant improvement even during the T2 vs. T3 period (p = 0.0028), despite no further meaningful changes being observed in other variables during this interval (Table 2).

Table 2.
Friedman’s ANOVA results and Durbin–Conover pairwise comparison (p-values).
3.3. QoL
3.3.1. General Scores
The analysis of WHO-DTSQ and SF-36 scales demonstrates a general improvement in patient satisfaction with treatment, summarized in Table 3. For SF-36, nearly all domains showed a noticeable increase in mean scores from T0 to T3, indicating an overall enhancement in QoL. However, certain domains exhibited fluctuations over time. For example, in Domain 2 (role limitations due to physical health problems), mean scores increased from 75.36 at T0 to 80.56 at T2 but decreased slightly to 76.79 at T3. In Domain 3 (Bodily Pain), scores improved from 69.08 at T0 to 78.24 at T1; then, they fluctuated at 74.4 (T2) and 77.78 (T3). Domain 7 (role limitations due to emotional health problems) varied from 69.24 at T0 to 68.13 (T1), 67.28 (T2), and 72.37 at T3. Domain 8 (Mental Health) showed a gradual increase from 46.01 at T0 to 51.92 at T3, despite minor fluctuations at intermediate time points (49.86 at T1 and 49.38 at T2). For the WHO-DTSQ, trends were more variable, with certain items fluctuating across the follow-up periods. For example, Item 1 (How satisfied are you with your current treatment?) fluctuated across follow-ups, while Items 2 and 3 (How often did you feel that your blood sugar was too high/low?) showed variability during the study period. Item 5 (How flexible did you find your treatment in the recent period?) and Item 6 (How satisfied are you with your knowledge of your diabetes?) also showed variability. Item 7 (Would you recommend your form of treatment to someone else?) fluctuated over time. Notably, Item 4 (How convenient/comfortable did you find your treatment?) and Item 8 (How satisfied would you be to continue with the current form of treatment?) displayed a steady, gradual improvement across all follow-ups, reflecting consistent satisfaction with treatment convenience and continuity (Table 3).

Table 3.
Mean and median scores for SF-36 and WHO-DTSQ.
3.3.2. Time Evolution of QoL
In the comparison of periods for the SF-36 questionnaire, no statistically significant differences were observed, except in the general health domain between T0 and T2 (p = 0.031). While not statistically significant, improvements were noted between the first follow-up (T0 vs. T1) and the last follow-up (T0 vs. T3) for Domains 1 (Physical Functioning) and 6 (Social Functioning). Regarding the WHO-DTSQ questionnaire, several items demonstrated statistically significant results: Item 4 (“How convenient/comfortable did you find your treatment in the recent period?”) showed significance across T0 vs. T1 (p = 0.021), T0 vs. T2 (p = 0.008), and T0 vs. T3 (p = 0.012); Item 5 (“How flexible did you find your treatment in the recent period?”) was significant for T0 vs. T1 (p = 0.021) and T0 vs. T2 (p = 0.021); Item 7 (“Would you recommend your form of treatment to someone else?”) achieved statistical significance for T0 vs. T1 (p < 0.001), T0 vs. T2 (p = 0.004), and T0 vs. T3 (p < 0.001); and Item 8 (“How satisfied would you be to continue with the current form of treatment?”) reached statistical significance for T0 vs. T1 (p < 0.001), T0 vs. T2 (p = 0.001), and T0 vs. T3 (p = 0.001) (Table 4).

Table 4.
SF-36 and WHO-DTSQ domain’s score Friedman’s ANOVA and Durbin–Conover’s pairwise comparison p-value.
4. Discussion
4.1. Effects of Oral Semaglutide on Clinical Outcomes
This study investigated the impact of oral semaglutide on HbA1c, FPG, body composition, anthropometric measurements, cardiovascular risk factors, and QoL in individuals with T2D over an 18-month treatment period. The results indicate that the daily administration of oral semaglutide (14 mg/day) yields significant benefits in glycemic control, weight reduction, and lipid profile (LP), along with improvements in patient-reported outcomes such as satisfaction and QoL. A particularly noteworthy finding was the pronounced decrease in HbA1c from baseline (T0) to the final assessment (T3), with a statistically significant correlation during the T2 to T3 period (p = 0.0028). This outcome underscores semaglutide’s capacity to maintain long-term glycemic control, which plays a critical role in reducing the likelihood of diabetes-related complications [46]. The existing literature has consistently shown that lowering HbA1c levels is associated with decreased risks of both microvascular and macrovascular complications in patients with T2D [47]. The sustained HbA1c reduction observed (p < 0.001, T0 vs. T3) further supports semaglutide as an effective long-term therapeutic option. Concurrent reductions in FPG over the course of treatment reinforce semaglutide’s role in stabilizing daily blood glucose levels, which is vital for avoiding acute hyperglycemic events and ensuring consistent glycemic management [48]. These findings affirm the utility of GLP-1 receptor agonists in regulating everyday glucose fluctuations. Weight control is a fundamental aspect of T2D treatment, and our data revealed significant declines in both body weight and BMI across the follow-up phases, except during the T2 to T3 interval. Given that excess weight and obesity are primary contributors to the development and progression of T2D, effective weight loss is essential to minimize these risks [49,50,51]. The reductions in body weight and BMI observed in this study are consistent with previous evidence supporting the weight-loss efficacy of GLP-1 receptor agonists in patients with T2D [52]. Addressing cardiovascular risk is also essential in managing T2D, given the high prevalence of CVD among individuals with diabetes [53]. In our cohort, significant decreases in total TC and LDL-C were observed, both of which are central targets in cardiovascular risk management. Lowering LDL-C is a key strategy in reducing cardiovascular morbidity and mortality among patients with T2D [54,55]. The lipid-lowering effects associated with semaglutide align with prior studies and meta-analyses that have demonstrated the efficacy of GLP-1 receptor agonists in improving LP and mitigating cardiovascular risk [56,57]. Although previous findings have suggested that GLP-1 receptor agonists may also help lower BP [58,59,60], no statistically significant changes in systolic or diastolic BP were recorded in our study. This discrepancy might stem from individual variability, baseline BP stability, or study design factors. Further research is necessary to better understand the role of semaglutide in BP regulation, especially among hypertensive subpopulations.
4.2. Impact on QoL and Treatment Satisfaction
Beyond the clinical and metabolic improvements documented, this study also emphasizes the beneficial effects of oral semaglutide on patients’ QoL and overall satisfaction with their treatment regimen, as measured through the SF-36 [38] and WHO-DTSQ questionnaires [39]. The observed enhancements across QoL domains indicate a more comprehensive improvement in patients’ physical, emotional, and social functioning over the course of therapy. For instance, although changes in physical functioning and social functioning scores were not statistically significant, the positive trends suggest that oral semaglutide may help alleviate the daily challenges and social limitations often experienced by individuals with T2D, who frequently struggle with disease-related demands and complications. Prior studies have found that GLP-1 receptor agonists, including semaglutide, are associated with increased treatment satisfaction and perceived effectiveness [61]. The PIONEER 3 trial, for example, reported greater satisfaction among patients using oral semaglutide when compared to other antidiabetic therapies [62]. Our SF-36 findings further reflected this pattern, with variable yet encouraging improvements in domains such as role limitations due to physical or emotional problems, as well as general health perception. However, the lack of significant change in perceived general health may stem from the complex, multifactorial nature of QoL, which extends beyond measurable changes in glycemic status or weight [63]. This aligns with the understanding that short-to-medium-term clinical improvements do not always immediately result in substantial shifts in perceived overall well-being. Findings from the WHO-DTSQ provided further insight, showing significant gains in treatment-related convenience and flexibility, alongside a greater willingness to continue with the current therapeutic approach. These dimensions are especially important in enhancing treatment adherence—a central factor in long-term disease management for chronic conditions such as T2D [64,65]. Specifically, Items 4 and 8 of the WHO-DTSQ, which assess perceived convenience and willingness to maintain treatment, demonstrated consistent and statistically significant improvement across all evaluation points. These outcomes highlight how the oral formulation of semaglutide, offering an alternative to injectable treatments, may play a crucial role in improving patient engagement and persistence in real-world therapeutic contexts, particularly among those hesitant to initiate injectable therapies. Taken together, these results indicate that oral semaglutide not only facilitates effective glycemic control and weight loss but also contributes meaningfully to improving QoL and treatment satisfaction in people living with T2D. Such multidimensional benefits are essential in chronic disease management, where sustaining patient motivation and adherence over time remains a major challenge. By simultaneously enhancing objective metabolic outcomes and subjective patient experiences, oral semaglutide meets several core goals of modern diabetes care and supports its expanded use in routine clinical practice.
4.3. Perspective for Clinical Practice in Public Health View
T2D and obesity are critical public health issues linked to increased CVD and healthcare costs [66,67]. Oral semaglutide offers a valuable option by improving glycemic control, promoting weight loss, and enhancing patient satisfaction, all of which support better disease management at the population level [16]. Its oral formulation can increase treatment adherence by overcoming barriers common to injectable therapies, improving long-term outcomes [14,16]. Integrating oral semaglutide into public health strategies requires a coordinated, multidisciplinary approach where healthcare professionals collaborate within integrated networks, each contributing their specific expertise [68]. This teamwork should combine pharmacological treatment with lifestyle interventions and patient education [69,70,71,72]. Additionally, leveraging new digital health technologies—such as telemedicine, mobile apps, and remote monitoring—can further support chronic disease management in general and diabetes in particular by enabling personalized care, improving adherence, and facilitating timely interventions and self-education [73,74,75,76,77,78]. These combined efforts are essential to address the complex challenges of diabetes and obesity and reduce their burden on healthcare.
4.4. Limitations
This study has several limitations that affect the generalizability of its findings. First, the sample size was limited to 97 adult patients, all recruited from two similar centers and sharing relatively homogeneous clinical characteristics. This restricts the applicability of results to broader populations, particularly those with different ethnic, socioeconomic, or comorbid profiles. Future research should include multi-center trials with larger and more diverse cohorts to ensure findings are representative of the general T2D population. Second, the 18-month follow-up, although adequate for capturing short- to medium-term outcomes, does not allow conclusions to be drawn about the long-term sustainability of the benefits associated with oral semaglutide over extended periods. Prolonged observational periods are needed to assess whether improvements in glycemic control, weight, cardiovascular risk, and QoL persist over time. Additionally, the lack of a placebo or active comparator group limits the internal validity of the study, making it difficult to isolate the effects of the intervention from other influencing factors. Randomized controlled designs are essential in future studies to establish causality more robustly. The use of self-reported instruments for QoL and treatment satisfaction may also introduce bias. Incorporating objective assessments alongside self-reported outcomes would provide a more comprehensive evaluation. Moreover, potential confounding factors—such as variability in adherence to dietary recommendations, exercise habits, and concomitant medications—were not tightly controlled, which could have influenced the results. The absence of a control arm with injectable semaglutide limits the ability to perform a direct and meaningful comparison regarding the acceptability and appreciation of the oral formulation. Future research should implement more rigorous control of these variables. Finally, body composition was assessed using BIA, which, despite being practical and non-invasive, lacks the precision of more advanced methods such as dual-energy X-ray absorptiometry (DEXA). Employing more accurate techniques in future studies would enhance the reliability of body composition data.
5. Conclusions
This study demonstrates that oral semaglutide (14 mg daily) effectively improves glycemic control, promotes weight loss, and reduces cardiovascular risk factors in T2D patients. Given the global rise in diabetes and obesity, such therapies are vital for addressing these interconnected public health challenges. The oral formulation enhances treatment adherence by offering a convenient alternative to injectables therapies, which is crucial for the long-term management of chronic conditions. Optimizing outcomes requires a coordinated, multidisciplinary approach, in which healthcare professionals collaborate within integrated networks, each contributing their expertise to support patients comprehensively. Combining semaglutide with lifestyle changes, digital health tools, and team-based care can further improve diabetes management and reduce its burden on healthcare systems. While longer-term and larger-scale studies are still needed, oral semaglutide represents a promising option aligned with both clinical and public health priorities.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diabetology6080080/s1, S1. STROBE Statement—checklist; S2. Italian versions of the Short Form Health Survey (SF-36); S3. WHO-diabetes treatment satisfaction questionnaire (WHO-DTSQ).
Author Contributions
Conceptualization, G.C. (Giovanni Cangelosi) and F.P.; methodology, G.C. (Giovanni Cangelosi) and S.M.; software, G.C. (Giovanni Cangelosi), G.M. and M.P.; validation, S.M. and G.C. (Gabriele Caggianelli); formal analysis, G.C. (Giovanni Cangelosi), S.M.P., S.T., G.B., F.G. and V.M.; investigation, G.C. (Gabriele Caggianelli), S.C., S.D.M., O.P., S.A. and C.D.C.; data curation, G.C. (Giovanni Cangelosi), G.M. and M.P.; writing—original draft preparation, G.C. (Giovanni Cangelosi), S.M., S.M.P., G.B., F.P. and S.M.P.; research, C.D.C., S.A., O.P., S.D.M., G.M., S.C., V.M., F.G., G.B. and S.T.; writing—review and editing, P.P., V.R., G.C. (Giovanni Cangelosi), S.M.P., S.M. and F.P.; visualization, P.P., V.R., S.M., C.D.C., S.A., O.P., G.M., S.C., S.D.M., V.M., F.G., G.B., S.T., S.M.P., F.P. and M.P.; supervision, G.C. (Giovanni Cangelosi) and F.P.; project administration, P.P., V.R., G.C. (Gabriele Caggianelli), G.C. (Giovanni Cangelosi), F.P., P.P. and V.R. contributed equally as first authors; M.P. and G.C. (Giovanni Cangelosi) contributed equally as second authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study adhered to the principles outlined in the Helsinki Declaration. Ethical approbation was performed CERM Ethics Committee (protocol number 2022/123-8040) on 17 October 2024.
Informed Consent Statement
All participants were informed about the study’s objectives, and the consent was obtained in compliance with all privacy regulations (Art. 13 EU Regulation 679/2016) before survey administration. The data were processed anonymously.
Data Availability Statement
The data analyzed in this study were provided in aggregated form by corporate sources and cannot be publicly shared due to ethical, contractual, and legal constraints. Specific data access requests may be considered on a case-by-case basis, subject to authorization by the relevant companies. The corresponding authors may act as intermediaries to facilitate contact with the data owners and assess the feasibility of such requests, in full compliance with applicable regulations.
Conflicts of Interest
The authors declare no competing interests.
References
- Ahmad, E.; Lim, S.; Lamptey, R.; Webb, D.R.; Davies, M.J. Type 2 diabetes. Lancet 2022, 400, 1803–1820. [Google Scholar] [CrossRef]
- Word Health Organization (WHO). The Global Diabetes Compact: Progress in Supporting its Workstreams: Technical Report. 2024. Available online: https://www.who.int/publications/i/item/9789240103092 (accessed on 23 December 2024).
- Word Health Organization (WHO). Guidance on Global Monitoring for Diabetes Prevention and Control: Framework, Indicators and Application. 2024. Available online: https://www.who.int/publications/i/item/9789240102248 (accessed on 23 December 2024).
- Tönnies, T.; Rathmann, W.; Hoyer, A.; Brinks, R.; Kuss, O. Quantifying the underestimation of projected global diabetes prevalence by the International Diabetes Federation (IDF) Diabetes Atlas. BMJ Open Diabetes Res. Care 2021, 9, e002122. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021, and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Istituto Nazionale di Statistica (ISTAT). Il Diabete in Italia. Available online: https://www.istat.it/it/archivio/202600 (accessed on 23 December 2024).
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022, 45, 2753–2786. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes—2024. Diabetes Care 2024, 47 (Suppl. S1), S158–S178. [Google Scholar] [CrossRef]
- Buse, J.B.; Wexler, D.J.; Tsapas, A.; Rossing, P.; Mingrone, G.; Mathieu, C.; D’alessio, D.A.; Davies, M.J. 2019, update to: Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2020, 63, 221–228. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 10. Cardiovascular Disease and Risk Management: Standards of Care in Diabetes—2024. Diabetes Care 2024, 47 (Suppl. S1), S179–S218. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 11. Chronic Kidney Disease and Risk Management: Standards of Care in Diabetes—2024. Diabetes Care 2024, 47 (Suppl. S1), S219–S230. [Google Scholar] [CrossRef]
- Lin, D.S.; Lee, J.K.; Hung, C.S.; Chen, W.J. The efficacy and safety of novel classes of glucose-lowering drugs for cardiovascular outcomes: A network meta-analysis of randomised clinical trials. Diabetologia 2021, 64, 2676–2686. [Google Scholar] [CrossRef]
- Brønden, A.; Christensen, M.B.; Glintborg, D.; Snorgaard, O.; Kofoed-Enevoldsen, A.; Madsen, G.K.; Toft, K.; Kristensen, J.K.; Højlund, K.; Hansen, T.K.; et al. Effects of DPP-4 inhibitors, GLP-1 receptor agonists, SGLT-2 inhibitors and sulphonylureas on mortality, cardiovascular and renal outcomes in type 2 diabetes: A network meta-analyses-driven approach. Diabetes Med. 2023, 40, e15157. [Google Scholar] [CrossRef]
- Pantanetti, P.; Cangelosi, G.; Alberti, S.; Di Marco, S.; Michetti, G.; Cerasoli, G.; Di Giacinti, M.; Coacci, S.; Francucci, N.; Petrelli, F.; et al. Changes in body weight and composition, metabolic parameters, and quality of life in patients with type 2 diabetes treated with subcutaneous semaglutide in real-world clinical practice. Front. Endocrinol. 2024, 15, 1394506. [Google Scholar] [CrossRef]
- Saravanan, P.; Bell, H.; Braae, U.C.; Collins, E.; Deinega, A.; Dhatariya, K.; Machell, A.; Trent, A.; Strzelecka, A. PIONEER REAL UK: A Multi-Centre, Prospective, Real-World Study of Once-Daily Oral Semaglutide Use in Adults with Type 2 Diabetes. Adv. Ther. 2024, 41, 4266–4281. [Google Scholar] [CrossRef]
- Pantanetti, P.; Ronconi, V.; Sguanci, M.; Palomares, S.M.; Mancin, S.; Tartaglia, F.C.; Cangelosi, G.; Petrelli, F. Oral Semaglutide in Type 2 Diabetes: Clinical-Metabolic Outcomes and Quality of Life in Real-World Practice. J. Clin. Med. 2024, 13, 4752. [Google Scholar] [CrossRef]
- van Houtum, W.; Schrömbges, P.; Amadid, H.; van Bon, A.C.; Braae, U.C.; Hoogstraten, C.; Herrings, H. Real-World Use of Oral Semaglutide in Adults with Type 2 Diabetes in the PIONEER REAL Netherlands Multicentre, Prospective, Observational Study. Diabetes Ther. 2024, 15, 1749–1768. [Google Scholar] [CrossRef]
- Buckley, S.T.; Bækdal, T.A.; Vegge, A.; Maarbjerg, S.J.; Pyke, C.; Ahnfelt-Rønne, J.; Madsen, K.G.; Scheele, S.G.; Alanentalo, T.; Kirk, R.K.; et al. Transcellular stomach absorption of a derivatized glucagon-like peptide-1 receptor agonist. Sci. Transl. Med. 2018, 10, eaar7047. [Google Scholar] [CrossRef]
- Baekdal, T.A.; Donsmark, M.; Hartoft-Nielsen, M.L.; Søndergaard, F.L.; Connor, A. Relationship Between Oral Semaglutide Tablet Erosion and Pharmacokinetics: A Pharmacoscintigraphic Study. Clin. Pharmacol. Drug Dev. 2021, 10, 453–462. [Google Scholar] [CrossRef]
- García-Pérez, L.E.; Alvarez, M.; Dilla, T.; Gil-Guillén, V.; Orozco-Beltrán, D. Adherence to therapies in patients with type 2 diabetes. Diabetes Ther. 2013, 4, 175–194. [Google Scholar] [CrossRef]
- Tiktin, M.; Celik, S.; Berard, L. Understanding adherence to medications in type 2 diabetes care and clinical trials to overcome barriers: A narrative review. Curr. Med. Res. Opin. 2016, 32, 277–287. [Google Scholar] [CrossRef]
- Losi, S.; Berra, C.C.F.; Fornengo, R.; Pitocco, D.; Biricolti, G.; Federici, M.O. The role of patient preferences in adherence to treatment in chronic disease: A narrative review. Drug Target. Insights 2021, 15, 13–20. [Google Scholar] [CrossRef]
- Hauber, B.; Hand, M.V.; Hancock, B.C.; Zarrella, J.; Harding, L.; Ogden-Barker, M.; Antipas, A.S.; Watt, S.J. Patient Acceptability and Preferences for Solid Oral Dosage Form Drug Product Attributes: A Scoping Review. Patient Prefer. Adherence 2024, 18, 1281–1297. [Google Scholar] [CrossRef]
- Aroda, V.R.; Rosenstock, J.; Terauchi, Y.; Altuntas, Y.; Lalic, N.M.; Villegas, E.C.M.; Jeppesen, O.K.; Christiansen, E.; Hertz, C.L.; Haluzík, M. PIONEER 1: Randomized Clinical Trial of the Efficacy and Safety of Oral Semaglutide Monotherapy in Comparison with Placebo in Patients with Type 2 Diabetes. Diabetes Care 2019, 42, 1724–1732. [Google Scholar] [CrossRef]
- Rodbard, H.W.; Rosenstock, J.; Canani, L.H.; Deerochanawong, C.; Gumprecht, J.; Lindberg, S.; Lingvay, I.; Søndergaard, A.L.; Treppendahl, M.B.; Montanya, E. Oral Semaglutide versus Empagliflozin in Patients with Type 2 Diabetes Uncontrolled on Metformin: The PIONEER 2 Trial. Diabetes Care 2019, 42, 2272–2281. [Google Scholar] [CrossRef]
- Ji, L.; Agesen, R.M.; Bain, S.C.; Fu, F.; Gabery, S.; Geng, J.; Li, Y.; Lu, Y.; Luo, B.; Pang, W. Efficacy and safety of oral semaglutide vs sitagliptin in a predominantly Chinese population with type 2 diabetes uncontrolled with metformin: PIONEER 12, a double-blind, Phase IIIa, randomised trial. Diabetologia 2024, 67, 1800–1816. [Google Scholar] [CrossRef]
- Pratley, R.; Amod, A.; Hoff, S.T.; Kadowaki, T.; Lingvay, I.; Nauck, M.; Pedersen, K.B.; Saugstrup, T.; Meier, J.J. Oral Semaglutide versus Subcutaneous Liraglutide and Placebo in Type 2 Diabetes (PIONEER 4): A Randomised, Double-Blind, Phase 3a Trial. Lancet 2019, 394, 39–50. [Google Scholar] [CrossRef]
- Mosenzon, O.; Blicher, T.M.; Rosenlund, S.; Eriksson, J.W.; Heller, S.; Hels, O.H.; Pratley, R.; Sathyapalan, T.; Desouza, C.; Abramof, R.; et al. Efficacy and Safety of Oral Semaglutide in Patients with Type 2 Diabetes and Moderate Renal Impairment (PIONEER 5): A PlaceboControlled, Randomised, Phase 3a Trial. Lancet Diabetes Endocrinol. 2019, 7, 515–527. [Google Scholar] [CrossRef]
- Pieber, T.R.; Bode, B.; Mertens, A.; Cho, Y.M.; Christiansen, E.; Hertz, C.L.; Wallenstein, S.O.R.; Buse, J.B.; Akın, S.; Aladağ, N.; et al. Efficacy and Safety of Oral Semaglutide with Flexible Dose Adjustment versus Sitagliptin in Type 2 Diabetes (PIONEER 7): A Multicentre, OpenLabel, Randomised, Phase 3a Trial. Lancet Diabetes Endocrinol. 2019, 7, 528–539. [Google Scholar] [CrossRef]
- Zinman, B.; Aroda, V.R.; Buse, J.B.; Cariou, B.; Harris, S.B.; Hoff, S.T.; Pedersen, K.B.; Tarp-Johansen, M.J.; Araki, E. Efficacy, Safety, and Tolerability of Oral Semaglutide versus Placebo Added to Insulin with or without Metformin in Patients with Type 2 Diabetes: The PIONEER 8 Trial. Diabetes Care 2019, 42, 2262–2271. [Google Scholar] [CrossRef]
- Yamada, Y.; Katagiri, H.; Hamamoto, Y.; Deenadayalan, S.; Navarria, A.; Nishijima, K.; Seino, Y.; Fukushima, Y.; Hisatomi, A.; Ide, Y.; et al. Dose-Response, Efficacy, and Safety of Oral Semaglutide Monotherapy in Japanese Patients with Type 2 Diabetes (PIONEER 9): A 52-Week, Phase 2/3a, Randomised, Controlled Trial. Lancet Diabetes Endocrinol. 2020, 8, 377–391. [Google Scholar] [CrossRef]
- Yabe, D.; Nakamura, J.; Kaneto, H.; Deenadayalan, S.; Navarria, A.; Gislum, M.; Inagaki, N.; Arisaka, T.; Asakura, T.; Azuma, N.; et al. Safety and Efficacy of Oral Semaglutide versus Dulaglutide in Japanese Patients with Type 2 Diabetes (PIONEER 10): An Open-Label, Randomised, Active-Controlled, Phase 3a Trial. Lancet Diabetes Endocrinol. 2020, 8, 392–406. [Google Scholar] [CrossRef]
- Mansoor, H.; Birkenfeld, A.L.; Donsmark, M.; Dungan, K.; Eliaschewitz, F.G.; Franco, D.R.; Jeppesen, O.K.; Lingvay, I.; Mosenzon, O.; Pedersen, S.D.; et al. Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2019, 381, 841–851. [Google Scholar] [CrossRef]
- Andersen, A.; Knop, F.K.; Vilsboll, T. A Pharmacological and Clinical Overview of Oral Semaglutide for the Treatment of Type 2 Diabetes. Drugs 2021, 81, 1003–1030. [Google Scholar] [CrossRef]
- Yanai, H.; Hakoshima, M.; Adachi, H.; Katsuyama, H. A Significant Effect of Oral Semaglutide on Cardiovascular Risk Factors in Patients with Type 2 Diabetes. Cardiol. Res. 2022, 13, 303–308. [Google Scholar] [CrossRef]
- Klobučar, S.; Belančić, A.; Bukša, I.; Morić, N.; Rahelić, D. Effectiveness of Oral versus Injectable Semaglutide in Adults with Type 2 Diabetes: Results from a Retrospective Observational Study in Croatia. Diabetology 2023, 5, 60–68. [Google Scholar] [CrossRef]
- Candido, R.; Gaiotti, S.; Giudici, F.; Toffoli, B.; De Luca, F.; Velardi, V.; Petrucco, A.; Gottardi, C.; Manca, E.; Buda, I.; et al. Real-World Retrospective Study into the Effects of Oral Semaglutide (As a Switchover or Add-On Therapy) in Type 2 Diabetes. J. Clin. Med. 2023, 12, 6052. [Google Scholar] [CrossRef]
- Nicolucci, A.; Giorgino, R.; Cucinotta, D.; Zoppini, G.; Muggeo, M.; Squatrito, S.; Corsi, A.; Lostia, S.; Pappalardo, L.; Benaduce, E.; et al. Validation of the italian version of the WHO well-being questionnaire (WHO-WBQ) and the WHO-diabetes treatment satisfaction questionnaire (WHO-DTSQ). Diabetes Nutr. Metab. 2004, 17, 235–243. [Google Scholar]
- Apolone, G.; Mosconi, P. The Italian SF-36 Health Survey: Translation, validation and norming. J. Clin. Epidemiol. 1998, 51, 1025–1036. [Google Scholar] [CrossRef]
- Ishii, H.; Hansen, B.B.; Langer, J.; Horio, H. Effect of Orally Administered Semaglutide Versus Dulaglutide on Diabetes-Related Quality of Life in Japanese Patients with Type 2 Diabetes: The PIONEER 10 Randomized, Active-Controlled Trial. Diabetes Ther. 2021, 12, 613–623. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ 2007, 335, 806–808. [Google Scholar] [CrossRef]
- McHorney, C.A.; Ware, J.E.J.; Lu, J.F.R.; Sherbourne, C.D. The MOS 36-item shortform health survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med. Care 1994, 32, 40–66. [Google Scholar] [CrossRef]
- Musa, A.F.; Yasin, M.S.M.; Smith, J.; Yakub, M.A.; Nordin, R.B. The Malay version of SF-36 health survey instrument: Testing data quality, scaling assumptions, reliability and validity in post-coronary artery bypass grafting (CABG) surgery patients at the National Heart Institute (Institut Jantung Negara-IJN), Kuala Lumpur. Health Qual. Life Outcomes 2021, 19, 50. [Google Scholar] [CrossRef]
- Heymans, M.W.; Twisk, J.W.R. Handling missing data in clinical research. J. Clin. Epidemiol. 2022, 151, 185–188. [Google Scholar] [CrossRef]
- Kang, H. The prevention and handling of the missing data. Korean J. Anesthesiol. 2013, 64, 402–406. [Google Scholar] [CrossRef]
- Perais, J.; Agarwal, R.; Evans, J.R.; Loveman, E.; Colquitt, J.L.; Owens, D.; Hogg, R.E.; Lawrenson, J.G.; Takwoingi, Y.; Lois, N.; et al. Prognostic factors for the development and progression of proliferative diabetic retinopathy in people with diabetic retinopathy. Cochrane Database Syst. Rev. 2023, 2, CD013775. [Google Scholar] [CrossRef]
- Pei, J.; Wang, X.; Pei, Z.; Hu, X. Glycemic control, HbA1c variability, and major cardiovascular adverse outcomes in type 2 diabetes patients with elevated cardiovascular risk: Insights from the ACCORD study. Cardiovasc. Diabetol. 2023, 22, 287. [Google Scholar] [CrossRef]
- Cavero-Redondo, I.; Peleteiro, B.; Álvarez-Bueno, C.; Rodriguez-Artalejo, F.; Martínez-Vizcaíno, V. Glycated haemoglobin A1c as a risk factor of cardiovascular outcomes and all-cause mortality in diabetic and non-diabetic populations: A systematic review and meta-analysis. BMJ Open 2017, 7, e015949. [Google Scholar] [CrossRef]
- Cangelosi, G.; Acito, M.; Grappasonni, I.; Nguyen, C.T.T.; Tesauro, M.; Pantanetti, P.; Morichetti, L.; Ceroni, E.; Benni, A.; Petrelli, F. Yoga or Mindfulness on Diabetes: Scoping Review for Theoretical Experimental Framework. Ann. Ig. 2024, 36, 153–168. [Google Scholar] [CrossRef] [PubMed]
- White, L.; Kirwan, M.; Christie, V.; Hurst, L.; Gwynne, K. The Effectiveness of Clinician-Led Community-Based Group Exercise Interventions on Health Outcomes in Adults with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2024, 21, 601. [Google Scholar] [CrossRef]
- Petrelli, F.; Cangelosi, G.; Scuri, S.; Cuc Thi Thu, N.; Debernardi, G.; Benni, A.; Vesprini, A.; Rocchi, R.; De Carolis, C.; Pantanetti, P.; et al. Food knowledge of patients at the first access to a Diabetology center. Acta Biomed. 2020, 91, 160–164. [Google Scholar] [CrossRef]
- Moore, P.W.; Malone, K.; VanValkenburg, D.; Rando, L.L.; Williams, B.C.; Matejowsky, H.G.; Ahmadzadeh, S.; Shekoohi, S.; Cornett, E.M.; Kaye, A.D. GLP-1 Agonists for Weight Loss: Pharmacology and Clinical Implications. Adv. Ther. 2023, 40, 723–742. [Google Scholar] [CrossRef]
- White, G.E.; Shu, I.; Rometo, D.; Arnold, J.; Korytkowski, M.; Luo, J. Real-world weight-loss effectiveness of glucagon-like peptide-1 agonists among patients with type 2 diabetes: A retrospective cohort study. Obesity 2023, 31, 537–544. [Google Scholar] [CrossRef]
- Dal Canto, E.; Ceriello, A.; Rydén, L.; Ferrini, M.; Hansen, T.B.; Schnell, O.; Standl, E.; Beulens, J.W. Diabetes as a cardiovascular risk factor: An overview of global trends of macro and micro vascular complications. Eur. J. Prev. Cardiol. 2019, 26 (Suppl. S2), 25–32. [Google Scholar] [CrossRef]
- Hasheminasabgorji, E.; Jha, J.C. Dyslipidemia, Diabetes and Atherosclerosis: Role of Inflammation and ROS-Redox-Sensitive Factors. Biomedicines 2021, 9, 1602. [Google Scholar] [CrossRef]
- Poznyak, A.; Grechko, A.V.; Poggio, P.; Myasoedova, V.A.; Alfieri, V.; Orekhov, A.N. The Diabetes Mellitus-Atherosclerosis Connection: The Role of Lipid and Glucose Metabolism and Chronic Inflammation. Int. J. Mol. Sci. 2020, 21, 1835. [Google Scholar] [CrossRef]
- Rezaei, S.; Tabrizi, R.; Nowrouzi-Sohrabi, P.; Jalali, M.; Atkin, S.L.; Al-Rasadi, K.; Jamialahmadi, T.; Sahebkar, A. GLP-1 Receptor Agonist Effects on Lipid and Liver Profiles in Patients with Nonalcoholic Fatty Liver Disease: Systematic Review and Meta-Analysis. Can. J. Gastroenterol. Hepatol. 2021, 2021, 8936865. [Google Scholar] [CrossRef]
- Yao, H.; Zhang, A.; Li, D.; Wu, Y.; Wang, C.-Z.; Wan, J.-Y.; Yuan, C.-S. Comparative effectiveness of GLP-1 receptor agonists on glycaemic control, body weight, and lipid profile for type 2 diabetes: Systematic review and network meta-analysis. BMJ 2024, 384, e076410. [Google Scholar] [CrossRef]
- Husain, M.; Bain, S.C.; Jeppesen, O.K.; Lingvay, I.; Sorrig, R.; Treppendahl, M.B.; Vilsboll, T. Semaglutide (SUSTAIN and PIONEER) reduces cardiovascular events in type 2 diabetes across varying cardiovascular risk. Diabetes Obes. Metab. 2020, 22, 442–451. [Google Scholar] [CrossRef]
- Nauck, M.A.; Quast, D.R. Cardiovascular Safety and Benefits of Semaglutide in Patients with Type 2 Diabetes: Findings from SUSTAIN 6 and PIONEER 6. Front. Endocrinol. 2021, 12, 645566. [Google Scholar] [CrossRef]
- Aroda, V.R.; Faurby, M.; Lophaven, S.; Noone, J.; Wolden, M.L.; Lingvay, I. Insights into the early use of oral semaglutide in routine clinical practice: The IGNITE study. Diabetes Obes. Metab. 2021, 23, 2177–2182. [Google Scholar] [CrossRef] [PubMed]
- Rosenstock, J.; Allison, D.; Birkenfeld, A.L.; Blicher, T.M.; Deenadayalan, S.; Jacobsen, J.B.; Serusclat, P.; Violante, R.; Watada, H.; Davies, M. PIONEER 3 Investigators. Effect of Additional Oral Semaglutide vs Sitagliptin on Glycated Hemoglobin in Adults with Type 2 Diabetes Uncon-trolled With Metformin Alone or With Sulfonylurea: The PIONEER 3 Randomized Clinical Trial. JAMA 2019, 321, 1466–1480. [Google Scholar] [CrossRef]
- Billing, L.K.; Handelsman, Y.; Heile, M.; Schneider, D.; Wyne, K. Health-Related Quality of Life Assessments with Once-Weekly Glucagon-Like Peptide-1 Receptor Agonists in Type 2 Diabetes Mellitus. J. Manag. Care Spec. Pharm. 2018, 24 (9-a Suppl), S30–S41. [Google Scholar] [CrossRef]
- Majeed, A.; Rehman, M.; Hussain, I.; Imran, I.; Saleem, M.U.; Saeed, H.; Hashmi, F.K.; Akbar, M.; Abrar, M.A.; Ramzan, B.; et al. The Impact of Treatment Adherence on Quality of Life Among Type 2 Diabetes Mellitus Patients—Findings from a Cross-Sectional Study. Patient Prefer. Adherence 2021, 15, 475–481. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- Ezendu, K.; Pohl, G.; Lee, C.J.; Wang, H.; Li, X.; Dunn, J.P. Prevalence of Obesity-Related Multimorbidity and Its Health Care Costs among Adults in the United States. J. Manag. Care Spec. Pharm. 2025, 31, 179–188. [Google Scholar] [CrossRef]
- Marassi, M.; Fadini, G.P. Real-World Evidence on Oral Semaglutide for the Management of Type 2 Diabetes: A Narrative Review for Clinical Practice. Clin. Ther. 2025, 47, 102–110. [Google Scholar] [CrossRef]
- Jialal, I.; Olatunbosun, S.T. Oral Semaglutide Therapy Reduces Cardiovascular Events in Patients with Type 2 Diabetes: Deciphering the Soul of the Study. J. Clin. Med. 2025, 14, 3335. [Google Scholar] [CrossRef]
- Cangelosi, G.; Mancin, S.; Pantanetti, P.; Nguyen, C.T.T.; Morales Palomares, S.; Biondini, F.; Sguanci, M.; Petrelli, F. Lifestyle Medicine Case Manager Nurses for Type Two Diabetes Patients: An Overview of a Job Description Framework—A Narrative Review. Diabetology 2024, 5, 375–388. [Google Scholar] [CrossRef]
- Whitley, H.P.; Smith, W.D.; Hanson, C.; Parton, J.M. Interdisciplinary Speed Dating Augments Diabetes Self-Management Education and Support to Improve Health Outcomes. Patient Educ. Couns. 2020, 103, 2305–2311. [Google Scholar] [CrossRef]
- Alshowair, A.; Altamimi, S.; Alshahrani, S.; Almubrick, R.; Ahmed, S.; Tolba, A.; Alkawai, F.; Alruhaimi, F.; Alsafwani, E.; AlSuwailem, F.; et al. Effectiveness of Case Manager Led Multi-Disciplinary Team Approach on Glycemic Control Amongst T2DM Patients in Primary Care in Riyadh: A Retrospective Follow-Up Study. J. Prim. Care Community Health 2023, 14, 21501319231204592. [Google Scholar] [CrossRef]
- Sguanci, M.; Mancin, S.; Gazzelloni, A.; Diamanti, O.; Ferrara, G.; Morales Palomares, S.; Parozzi, M.; Petrelli, F.; Cangelosi, G. The Internet of Things in the Nutritional Management of Patients with Chronic Neurological Cognitive Impairment: A Scoping Review. Healthcare 2024, 13, 23. [Google Scholar] [CrossRef]
- Basharat, A.; Thayanithy, A.; Barnett-Cowan, M. A Scoping Review of Audiovisual Integration Methodology: Screening for Auditory and Visual Impairment in Younger and Older Adults. Front. Aging Neurosci. 2022, 13, 772112. [Google Scholar] [CrossRef]
- Greenwood, D.A.; Litchman, M.L.; Isaacs, D.; Blanchette, J.E.; Dickinson, J.K.; Hughes, A.; Colicchio, V.D.; Ye, J.; Yehl, K.; Todd, A.; et al. A New Taxonomy for Technology-Enabled Diabetes Self-Management Interventions: Results of an Umbrella Review. J. Diabetes Sci. Technol. 2022, 16, 812–824. [Google Scholar] [CrossRef] [PubMed]
- Kerr, D.; Ahn, D.; Waki, K.; Wang, J.; Breznen, B.; Klonoff, D.C. Digital Interventions for Self-Management of Type 2 Diabetes Mellitus: Systematic Literature Review and Meta-Analysis. J. Med. Internet Res. 2024, 26, e55757. [Google Scholar] [CrossRef]
- Pantanetti, P.; Cangelosi, G.; Morales Palomares, S.; Ferrara, G.; Biondini, F.; Mancin, S.; Caggianelli, G.; Parozzi, M.; Sguanci, M.; Petrelli, F. Real-World Life Analysis of a Continuous Glucose Monitoring and Smart Insulin Pen System in Type 1 Diabetes: A Cohort Study. Diabetology 2025, 6, 7. [Google Scholar] [CrossRef]
- Cranston, I.; Jamdade, V.; Liao, B.; Newson, R.S. Clinical, Economic, and Patient-Reported Benefits of Connected Insulin Pen Systems: A Systematic Literature Review. Adv. Ther. 2023, 40, 2015–2037. [Google Scholar] [CrossRef] [PubMed]
- Akturk, H.K.; Bindal, A. Advances in Diabetes Technology within the Digital Diabetes Ecosystem. J. Manag. Care Spec. Pharm. 2024, 30 (10-b Suppl), S7–S20. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).