Exploring the Epidemiologic Burden, Pathogenetic Features, and Clinical Outcomes of Primary Liver Cancer in Patients with Type 2 Diabetes Mellitus (T2DM) and Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A Scoping Review
Abstract
1. Background
1.1. Primary Liver Cancer in the Era of MASLD: Epidemiological Changes Guide the Etiological Shift
1.2. Primary Liver Cancer in MASLD: Impact of T2DM on Relative Occurrence Risk and Prognosis
1.3. Status of the Art and Aims of the Research
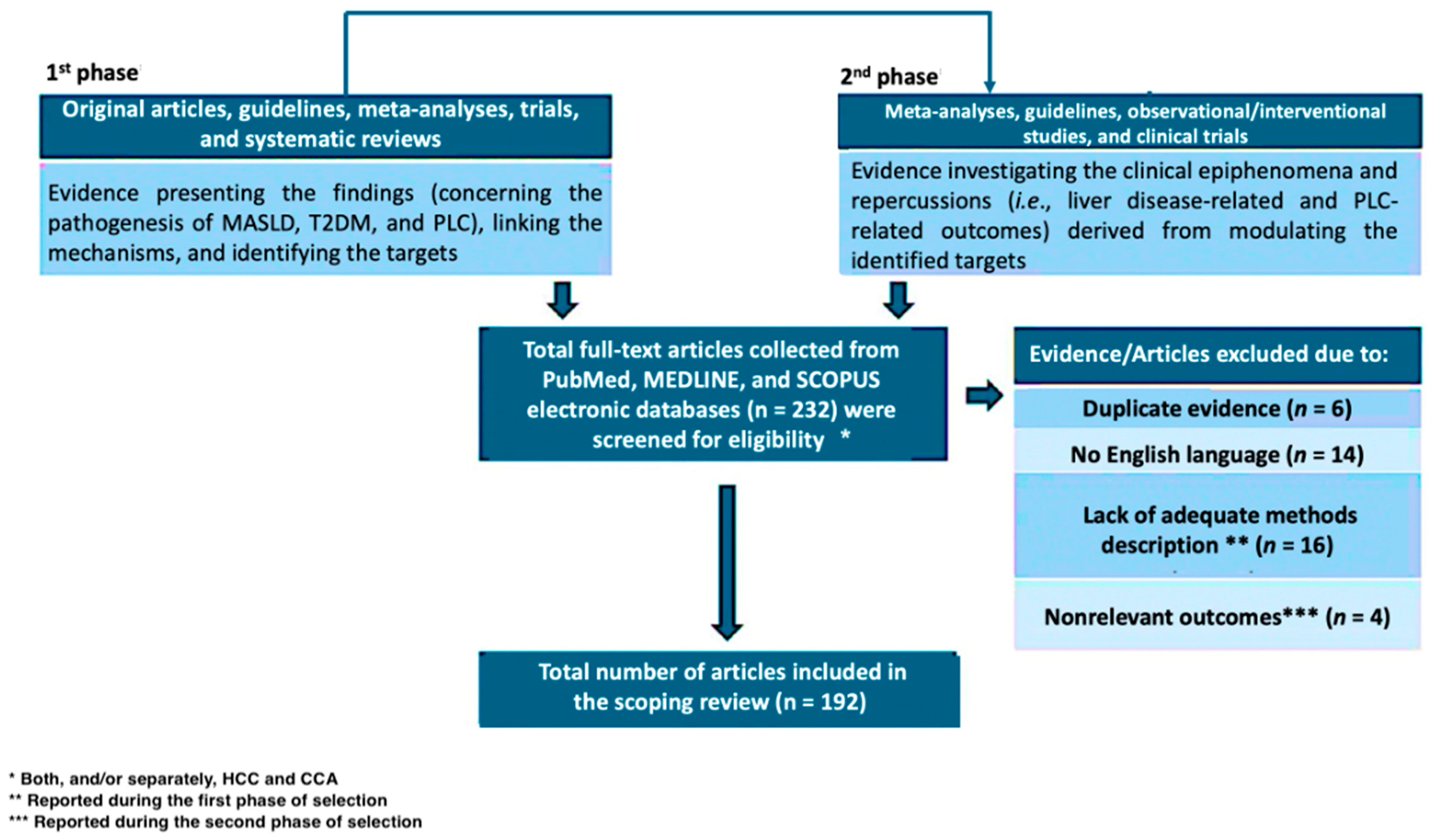
2. Methods
3. Results
3.1. Type 2 Diabetes Mellitus, MASLD, and Hepatic Cancer: From “Classic” to Novel Pathogenesis
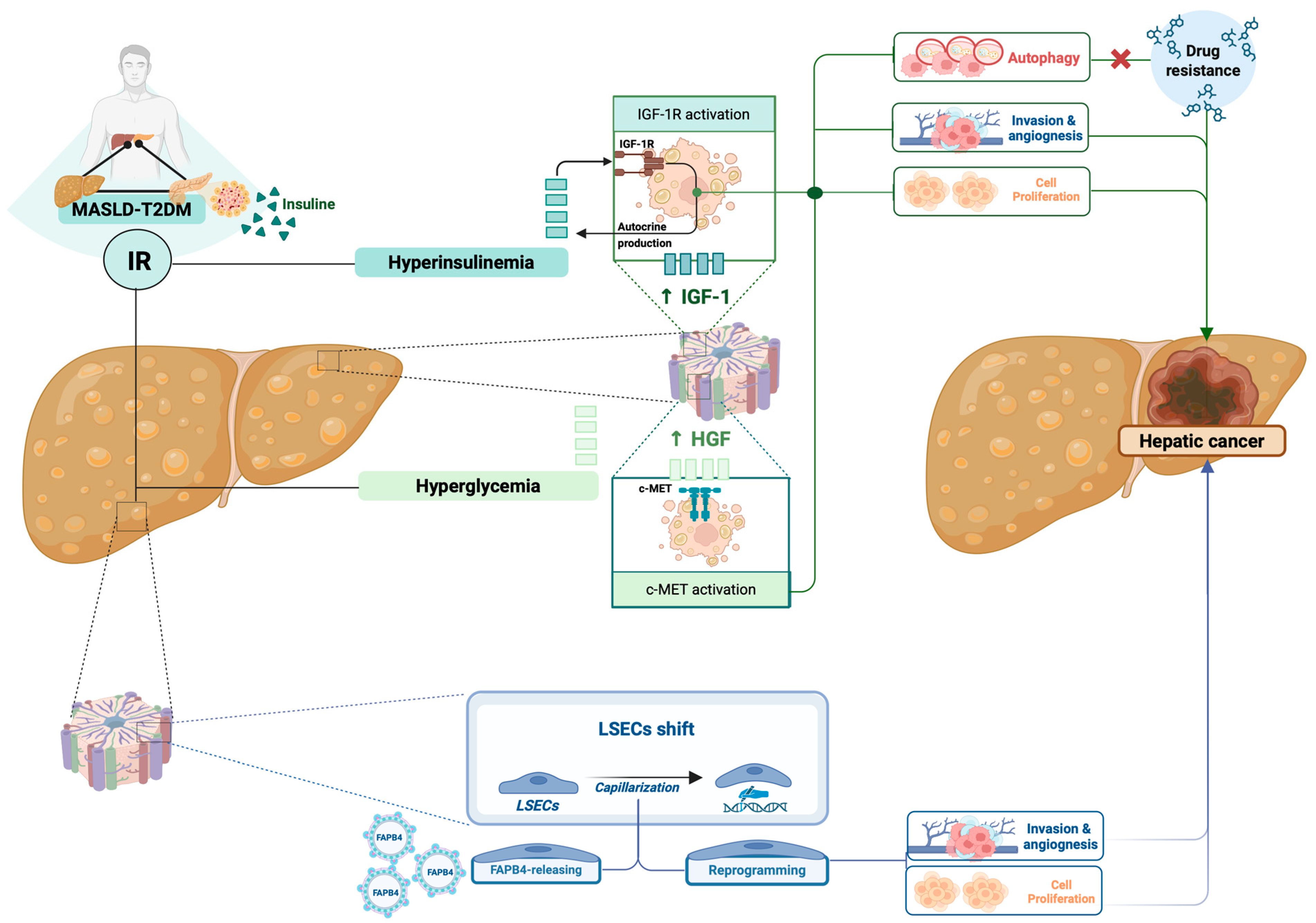
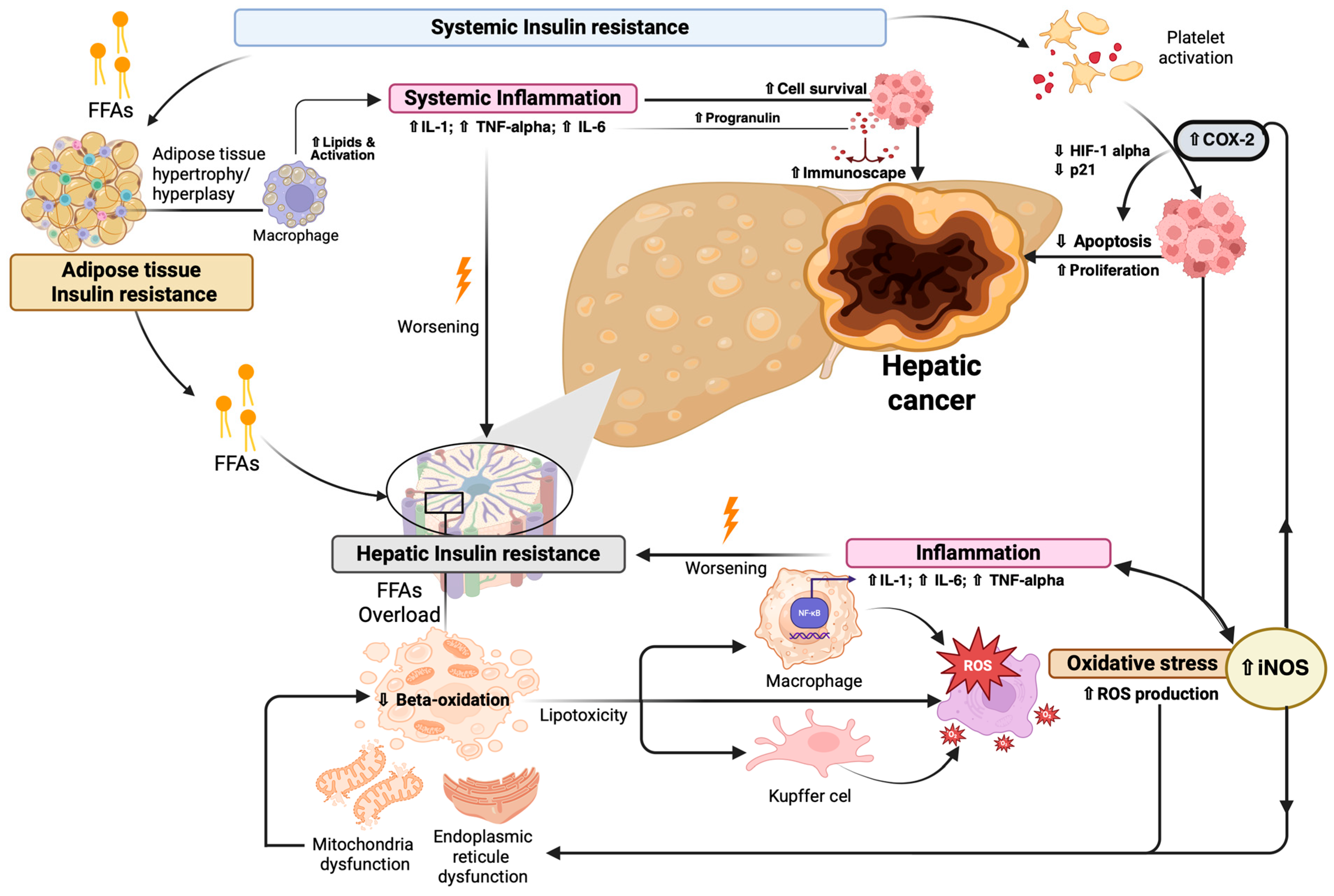
3.1.1. Role of Insulin Resistance in Influencing Hepatobiliary Cancerogenesis
3.1.2. Insulin Resistance-Related Inflammation Influences Hepatobiliary Cancerogenesis
3.1.3. MASLD-T2DM-Associated Gut Dysbiosis in the Pathogenesis of Primary Liver Cancer
3.1.4. Glucose-Related Metabolic Reprogramming Contributes to Cancer Immunoescape: The Emerging Frontiers of PLC Pathogenesis
3.2. Identifying Pathogenetic Targets to Design Tailored Strategies in the Management of MASLD-TD2M Associated PLC: State of the Art
3.2.1. Targeting Insulin Resistance
Metformin
SGLT2-Is
DPP4-Is
GLP-1 RAs
3.2.2. Modulating Gut-Biliary Liver Axis: A Promising and Still Embryonal Strategy
3.2.3. Modulating Immunometabolic Responses: The Novel Therapeutic Frontier
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AFP | Alpha-fetoprotein |
| AUD | Alcohol Use Disorder |
| BA | Bile Acid |
| BCG | Bacillus Calmette–Guérin |
| CCA | Cholangiocarcinoma |
| CMRF | Cardiometabolic Risk Factor |
| COX-2 | Cyclooxygenase-2 |
| DCA | Deoxycholic Acid |
| DM | Diabetes Mellitus |
| FABP4 | Fatty Acid Binding Protein 4 |
| FFAs | Free Fatty Acids |
| FLR | Future Liver Remnant |
| GLP-1 | Glucagon-Like Peptide-1 Receptor Agonists |
| HBV | Hepatitis B Virus |
| HCC | Hepatocellular Carcinoma |
| HCV | Hepatitis C Virus |
| HFD | High-Fat Diet |
| HGF | Hepatocyte Growth Factor |
| HR | Hazard Ratio |
| ICC | Intrahepatic Cholangiocarcinoma |
| ICIs | Immune Checkpoint Inhibitors |
| IGF-1 | Insulin-like Growth Factor 1 |
| IGF1-R | Insulin-like Growth Factor 1 Receptor |
| IGFBPs | Insulin-like Growth Factor-Binding Proteins |
| IL | Interleukin |
| IR | Insulin Resistance |
| JNK | c-Jun N-terminal Kinase |
| LD | Linear Dichroism |
| LPS | Lipopolysaccharide |
| LSECs | Liver Sinusoidal Endothelial Cells |
| MASLD | Metabolic Dysfunction-Associated Steatotic Liver Disease |
| MASH | Metabolic Dysfunction-Associated Steatohepatitis |
| MD | Metabolic Dysfunction |
| Met | MNNG HOS Transforming Gene |
| NAFLD | Non-Alcoholic Fatty Liver Disease |
| PDHC | Pyruvate Dehydrogenase Complex |
| PLC | Primary Liver Cancer |
| ROS s | Reactive Oxygen Species |
| SGLT2-Is | Sodium-Glucose Cotransporter-2 Inhibitors |
| SCFAs | Short-Chain Fatty Acids |
| STAT3 | STAT3—Signal Transducer and Activator of Transcription 3 |
| T2DM | T2DM—Type 2 Diabetes Mellitus |
| TCR | TCR—T-Cell Receptor |
| TGF-β | Transforming Growth Factor Beta |
| TI | Trained Immunity |
| TLR4 | Toll-like Receptor 4 |
| TNF-alpha | Tumor Necrosis Factor Alpha |
| TKI | Tyrosine Kinase Inhibitor |
| VEGF | Vascular Endothelial Growth Factor |
References
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73 (Suppl. S1), 4–13. [Google Scholar] [CrossRef]
- Rumgay, H.; Arnold, M.; Ferlay, J.; Lesi, O.; Cabasag, C.J.; Vignat, J.; Laversanne, M.; McGlynn, K.A.; Soerjomataram, I. Global Burden of Primary Liver Cancer in 2020 and Predictions to 2040. J. Hepatol. 2022, 77, 1598–1606. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y. Changing Etiology and Epidemiology of Hepatocellular Carcinoma: Asia and Worldwide. J. Liver Cancer 2024, 24, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhu, J.; Wang, G.; Groopman, J.D.; Kensler, T.W. Qidong: A Crucible for Studies on Liver Cancer Etiology and Prevention. Cancer Biol. Med. 2019, 16, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.-F.; Jeng, J.-E.; Chuang, L.-Y.; Ho, M.-S.; Ko, Y.-C.; Lin, Z.-Y.; Hsieh, M.-Y.; Chen, S.-C.; Chuang, W.-L.; Wang, L.-Y.; et al. Habitual Betel Quid Chewing as a Risk Factor for Cirrhosis: A Case-Control Study. Medicine 2003, 82, 365–372. [Google Scholar] [CrossRef]
- Maucort-Boulch, D.; de Martel, C.; Franceschi, S.; Plummer, M. Fraction and Incidence of Liver Cancer Attributable to Hepatitis B and C Viruses Worldwide. Int. J. Cancer 2018, 142, 2471–2477. [Google Scholar] [CrossRef]
- Florio, A.A.; Ferlay, J.; Znaor, A.; Ruggieri, D.; Alvarez, C.S.; Laversanne, M.; Bray, F.; McGlynn, K.A.; Petrick, J.L. Global Trends in Intrahepatic and Extrahepatic Cholangiocarcinoma Incidence from 1993 to 2012. Cancer 2020, 126, 2666–2678. [Google Scholar] [CrossRef]
- Qi, X.; Li, J.; Caussy, C.; Teng, G.-J.; Loomba, R. Epidemiology, Screening, and Co-Management of Type 2 Diabetes Mellitus and Metabolic Dysfunction-Associated Steatotic Liver Disease. Hepatology, 2024; online ahead of print. [Google Scholar] [CrossRef]
- Chan, W.-K.; Chuah, K.-H.; Rajaram, R.B.; Lim, L.-L.; Ratnasingam, J.; Vethakkan, S.R. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A State-of-the-Art Review. J. Obes. Metab. Syndr. 2023, 32, 197–213. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A Multisociety Delphi Consensus Statement on New Fatty Liver Disease Nomenclature. J. Hepatol. 2023, 79, 1542–1556. [Google Scholar] [CrossRef]
- Dallio, M.; Romeo, M.; Di Nardo, F.; Vaia, P.; Napolitano, C.; Ventriglia, L.; Coppola, A.; Silvestrin, A.; Olivieri, S.; Federico, A. FLAME: Training and Validating a Newly Conceived Model Incorporating Alpha-Glutathione-S-Transferase Serum Levels for Predicting Advanced Hepatic Fibrosis and Acute Cardiovascular Events in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Int. J. Mol. Sci. 2025, 26, 761. [Google Scholar] [CrossRef]
- Chrysavgis, L.; Cholongitas, E. From NAFLD to MASLD: What Does It Mean? Expert. Rev. Gastroenterol. Hepatol. 2024, 18, 217–221. [Google Scholar] [CrossRef]
- He, L.; Zheng, W.; Qiu, K.; Kong, W.; Zeng, T. Changing from NAFLD to MASLD: The New Definition Can More Accurately Identify Individuals at Higher Risk for Diabetes. J. Hepatol. 2024, 80, e85–e87. [Google Scholar] [CrossRef]
- De, A.; Bhagat, N.; Mehta, M.; Taneja, S.; Duseja, A. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) Definition Is Better than MAFLD Criteria for Lean Patients with NAFLD. J. Hepatol. 2024, 80, e61–e62. [Google Scholar] [CrossRef]
- Hagström, H.; Shang, Y.; Hegmar, H.; Nasr, P. Natural History and Progression of Metabolic Dysfunction-Associated Steatotic Liver Disease. Lancet Gastroenterol. Hepatol. 2024, 9, 944–956. [Google Scholar] [CrossRef] [PubMed]
- Phoolchund, A.G.S.; Khakoo, S.I. MASLD and the Development of HCC: Pathogenesis and Therapeutic Challenges. Cancers 2024, 16, 259. [Google Scholar] [CrossRef] [PubMed]
- Wongjarupong, N.; Assavapongpaiboon, B.; Susantitaphong, P.; Cheungpasitporn, W.; Treeprasertsuk, S.; Rerknimitr, R.; Chaiteerakij, R. Non-Alcoholic Fatty Liver Disease as a Risk Factor for Cholangiocarcinoma: A Systematic Review and Meta-Analysis. BMC Gastroenterol. 2017, 17, 149. [Google Scholar] [CrossRef] [PubMed]
- Onikanni, S.A.; Lawal, B.; Bakare, O.S.; Ajiboye, B.O.; Ojo, O.A.; Farasani, A.; Kabrah, S.M.; Batiha, G.E.-S.; Conte-Junior, C.A. Cancer of the Liver and Its Relationship with Diabetes Mellitus. Technol. Cancer Res. Treat. 2022, 21, 15330338221119743. [Google Scholar] [CrossRef]
- Chen, H.-P.; Shieh, J.-J.; Chang, C.-C.; Chen, T.-T.; Lin, J.-T.; Wu, M.-S.; Lin, J.-H.; Wu, C.-Y. Metformin Decreases Hepatocellular Carcinoma Risk in a Dose-Dependent Manner: Population-Based and in Vitro Studies. Gut 2013, 62, 606–615. [Google Scholar] [CrossRef]
- Jing, W.; Jin, G.; Zhou, X.; Zhou, Y.; Zhang, Y.; Shao, C.; Liu, R.; Hu, X. Diabetes Mellitus and Increased Risk of Cholangiocarcinoma: A Meta-Analysis. Eur. J. Cancer Prev. 2012, 21, 24–31. [Google Scholar] [CrossRef]
- Guo, Q.; Zhu, X.; Beeraka, N.M.; Zhao, R.; Li, S.; Li, F.; Mahesh, P.A.; Nikolenko, V.N.; Fan, R.; Liu, J. Projected Epidemiological Trends and Burden of Liver Cancer by 2040 Based on GBD, CI5plus, and WHO Data. Sci. Rep. 2024, 14, 28131. [Google Scholar] [CrossRef]
- Danpanichkul, P.; Aboona, M.B.; Sukphutanan, B.; Kongarin, S.; Duangsonk, K.; Ng, C.H.; Muthiah, M.D.; Huang, D.Q.; Seko, Y.; Díaz, L.A.; et al. Incidence of Liver Cancer in Young Adults According to the Global Burden of Disease Database 2019. Hepatology 2024, 80, 828–843. [Google Scholar] [CrossRef]
- Petrick, J.L.; Thistle, J.E.; Zeleniuch-Jacquotte, A.; Zhang, X.; Wactawski-Wende, J.; Van Dyke, A.L.; Stampfer, M.J.; Sinha, R.; Sesso, H.D.; Schairer, C.; et al. Body Mass Index, Diabetes and Intrahepatic Cholangiocarcinoma Risk: The Liver Cancer Pooling Project and Meta-Analysis. Am. J. Gastroenterol. 2018, 113, 1494–1505. [Google Scholar] [CrossRef]
- El-Serag, H.B.; Rudolph, K.L. Hepatocellular Carcinoma: Epidemiology and Molecular Carcinogenesis. Gastroenterology 2007, 132, 2557–2576. [Google Scholar] [CrossRef] [PubMed]
- Sohn, W.; Lee, H.W.; Lee, S.; Lim, J.H.; Lee, M.W.; Park, C.H.; Yoon, S.K. Obesity and the Risk of Primary Liver Cancer: A Systematic Review and Meta-Analysis. Clin. Mol. Hepatol. 2021, 27, 157–174. [Google Scholar] [CrossRef] [PubMed]
- Dallio, M.; Sangineto, M.; Romeo, M.; Cipullo, M.; Coppola, A.; Mammone, S.; Di Gioia, G.; Masarone, M.; Persico, M.; Serviddio, G.; et al. The Influence of Acute Lifestyle Changes on NAFLD Evolution in a Multicentre Cohort: A Matter of Body Composition. Nutr. Diabetes 2024, 14, 33. [Google Scholar] [CrossRef]
- Huang, D.Q.; Noureddin, N.; Ajmera, V.; Amangurbanova, M.; Bettencourt, R.; Truong, E.; Gidener, T.; Siddiqi, H.; Majzoub, A.M.; Nayfeh, T.; et al. Type 2 Diabetes, Hepatic Decompensation, and Hepatocellular Carcinoma in Patients with Non-Alcoholic Fatty Liver Disease: An Individual Participant-Level Data Meta-Analysis. Lancet Gastroenterol. Hepatol. 2023, 8, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Tsilidis, K.K.; Kasimis, J.C.; Lopez, D.S.; Ntzani, E.E.; Ioannidis, J.P.A. Type 2 Diabetes and Cancer: Umbrella Review of Meta-Analyses of Observational Studies. BMJ 2015, 350, g7607. [Google Scholar] [CrossRef]
- Li, J.; Han, T.; Xu, L.; Luan, X. Diabetes Mellitus and the Risk of Cholangiocarcinoma: An Updated Meta-Analysis. Prz. Gastroenterol. 2015, 10, 108–117. [Google Scholar] [CrossRef]
- Castera, L.; Cusi, K. Diabetes and Cirrhosis: Current Concepts on Diagnosis and Management. Hepatology 2023, 77, 2128–2146. [Google Scholar] [CrossRef]
- Wang, Y.-G.; Wang, P.; Wang, B.; Fu, Z.-J.; Zhao, W.-J.; Yan, S.-L. Diabetes Mellitus and Poorer Prognosis in Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e95485. [Google Scholar] [CrossRef]
- Takamatsu, S.; Noguchi, N.; Kudoh, A.; Nakamura, N.; Kawamura, T.; Teramoto, K.; Igari, T.; Arii, S. Influence of Risk Factors for Metabolic Syndrome and Non-Alcoholic Fatty Liver Disease on the Progression and Prognosis of Hepatocellular Carcinoma. Hepatogastroenterology 2008, 55, 609–614. [Google Scholar]
- Fu, K.; Yang, X.; Wu, H.; Gong, J.; Li, X. Diabetes and PKM2 Affect Prognosis in Patients with Intrahepatic Cholangiocarcinoma. Oncol. Lett. 2020, 20, 265. [Google Scholar] [CrossRef]
- Shibamoto, J.; Otsuka, S.; Okawa, Y.; Ashida, R.; Ohgi, K.; Kato, Y.; Dei, H.; Uesaka, K.; Sugiura, T. Prognostic Impact of Diabetes Mellitus and Extended Hepatectomy on Perihilar Cholangiocarcinoma. Ann. Surg. Open 2025, 6, e552. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, D.S.; An, T.H.; Park, H.-J.; Kim, W.K.; Bae, K.-H.; Oh, K.-J. Metabolic Spectrum of Liver Failure in Type 2 Diabetes and Obesity: From NAFLD to NASH to HCC. Int. J. Mol. Sci. 2021, 22, 4495. [Google Scholar] [CrossRef] [PubMed]
- Saengboonmee, C.; Seubwai, W.; Lert-Itthiporn, W.; Sanlung, T.; Wongkham, S. Association of Diabetes Mellitus and Cholangiocarcinoma: Update of Evidence and the Effects of Antidiabetic Medication. Can. J. Diabetes 2021, 45, 282–290. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO) EASL-EASD-EASO Clinical Practice Guidelines on the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). J. Hepatol. 2024, 81, 492–542. [CrossRef]
- Ferdous, S.-E.; Ferrell, J.M. Pathophysiological Relationship between Type 2 Diabetes Mellitus and Metabolic Dysfunction-Associated Steatotic Liver Disease: Novel Therapeutic Approaches. Int. J. Mol. Sci. 2024, 25, 8731. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Dallio, M.; Sangineto, M.; Romeo, M.; Villani, R.; Romano, A.D.; Loguercio, C.; Serviddio, G.; Federico, A. Immunity as Cornerstone of Non-Alcoholic Fatty Liver Disease: The Contribution of Oxidative Stress in the Disease Progression. Int. J. Mol. Sci. 2021, 22, 436. [Google Scholar] [CrossRef]
- Dallio, M.; Masarone, M.; Romeo, M.; Tuccillo, C.; Morisco, F.; Persico, M.; Loguercio, C.; Federico, A. PNPLA3, TM6SF2, and MBOAT7 Influence on Nutraceutical Therapy Response for Non-Alcoholic Fatty Liver Disease: A Randomized Controlled Trial. Front. Med. 2021, 8, 734847. [Google Scholar] [CrossRef]
- Romeo, M.; Dallio, M.; Scognamiglio, F.; Ventriglia, L.; Cipullo, M.; Coppola, A.; Tammaro, C.; Scafuro, G.; Iodice, P.; Federico, A. Role of Non-Coding RNAs in Hepatocellular Carcinoma Progression: From Classic to Novel Clinicopathogenetic Implications. Cancers 2023, 15, 5178. [Google Scholar] [CrossRef]
- Dallio, M.; Ventriglia, L.; Romeo, M.; Scognamiglio, F.; Diano, N.; Moggio, M.; Cipullo, M.; Coppola, A.; Ziogas, A.; Netea, M.G.; et al. Environmental Bisphenol A Exposure Triggers Trained Immunity-Related Pathways in Monocytes. Front. Immunol. 2023, 14, 1270391. [Google Scholar] [CrossRef] [PubMed]
- Palma, R.; Pronio, A.; Romeo, M.; Scognamiglio, F.; Ventriglia, L.; Ormando, V.M.; Lamazza, A.; Pontone, S.; Federico, A.; Dallio, M. The Role of Insulin Resistance in Fueling NAFLD Pathogenesis: From Molecular Mechanisms to Clinical Implications. J. Clin. Med. 2022, 11, 3649. [Google Scholar] [CrossRef] [PubMed]
- Crocetto, F.; Barone, B.; Manfredi, C.; Trama, F.; Romano, L.; Romeo, M.; Russo, G.; Sicignano, E.; Persico, F.; Aveta, A.; et al. Are Insulin Resistance and Non-Alcoholic Fatty Liver Disease Associated with Peyronie’s Disease? A Pilot Study. J. Physiol. Pharmacol. 2022, 73, 53–62. [Google Scholar] [CrossRef]
- Li, Y.; Yang, P.; Ye, J.; Xu, Q.; Wu, J.; Wang, Y. Updated Mechanisms of MASLD Pathogenesis. Lipids Health Dis. 2024, 23, 117. [Google Scholar] [CrossRef]
- Farrell, G. Insulin Resistance, Obesity, and Liver Cancer. Clin. Gastroenterol. Hepatol. 2014, 12, 117–119. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, J.; Xiao, W.; Fan, X. A Review of MASLD-Related Hepatocellular Carcinoma: Progress in Pathogenesis, Early Detection, and Therapeutic Interventions. Front. Med. 2024, 11, 1410668. [Google Scholar] [CrossRef]
- Kadowaki, T.; Tobe, K.; Honda-Yamamoto, R.; Tamemoto, H.; Kaburagi, Y.; Momomura, K.; Ueki, K.; Takahashi, Y.; Yamauchi, T.; Akanuma, Y.; et al. Signal Transduction Mechanism of Insulin and Insulin-like Growth Factor-1. Endocr. J. 1996, 43, S33–S41. [Google Scholar] [CrossRef]
- Scharf, J.-G.; Braulke, T. The Role of the IGF Axis in Hepatocarcinogenesis. Horm. Metab. Res. 2003, 35, 685–693. [Google Scholar] [CrossRef]
- Shan, Y.; Lu, C.; Wang, J.; Li, M.; Ye, S.; Wu, S.; Huang, J.; Bu, S.; Wang, F. IGF-1 Contributes to Liver Cancer Development in Diabetes Patients by Promoting Autophagy. Ann. Hepatol. 2022, 27, 100697. [Google Scholar] [CrossRef]
- Debnath, J.; Gammoh, N.; Ryan, K.M. Autophagy and Autophagy-Related Pathways in Cancer. Nat. Rev. Mol. Cell Biol. 2023, 24, 560–575. [Google Scholar] [CrossRef]
- Cai, W.; Ma, Y.; Song, L.; Cao, N.; Gao, J.; Zhou, S.; Tang, X. IGF-1R down Regulates the Sensitivity of Hepatocellular Carcinoma to Sorafenib through the PI3K/Akt and RAS / Raf / ERK Signaling Pathways. BMC Cancer 2023, 23, 87. [Google Scholar] [CrossRef]
- Ngo, M.-H.T.; Jeng, H.-Y.; Kuo, Y.-C.; Diony Nanda, J.; Brahmadhi, A.; Ling, T.-Y.; Chang, T.-S.; Huang, Y.-H. The Role of IGF/IGF-1R Signaling in Hepatocellular Carcinomas: Stemness-Related Properties and Drug Resistance. Int. J. Mol. Sci. 2021, 22, 1931. [Google Scholar] [CrossRef]
- Alvaro, D.; Barbaro, B.; Franchitto, A.; Onori, P.; Glaser, S.S.; Alpini, G.; Francis, H.; Marucci, L.; Sterpetti, P.; Ginanni-Corradini, S.; et al. Estrogens and Insulin-like Growth Factor 1 Modulate Neoplastic Cell Growth in Human Cholangiocarcinoma. Am. J. Pathol. 2006, 169, 877–888. [Google Scholar] [CrossRef]
- Manilla, V.; Santopaolo, F.; Gasbarrini, A.; Ponziani, F.R. Type 2 Diabetes Mellitus and Liver Disease: Across the Gut-Liver Axis from Fibrosis to Cancer. Nutrients 2023, 15, 2521. [Google Scholar] [CrossRef] [PubMed]
- Velliou, R.-I.; Legaki, A.-I.; Nikolakopoulou, P.; Vlachogiannis, N.I.; Chatzigeorgiou, A. Liver Endothelial Cells in NAFLD and Transition to NASH and HCC. Cell Mol. Life Sci. 2023, 80, 314. [Google Scholar] [CrossRef] [PubMed]
- Géraud, C.; Mogler, C.; Runge, A.; Evdokimov, K.; Lu, S.; Schledzewski, K.; Arnold, B.; Hämmerling, G.; Koch, P.S.; Breuhahn, K.; et al. Endothelial Transdifferentiation in Hepatocellular Carcinoma: Loss of Stabilin-2 Expression in Peri-Tumourous Liver Correlates with Increased Survival. Liver Int. 2013, 33, 1428–1440. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.; Dhoot, G.K. Dysregulated Cancer Cell Transdifferentiation into Erythrocytes Is an Additional Metabolic Stress in Hepatocellular Carcinoma. Tumour Biol. 2018, 40, 1010428318811467. [Google Scholar] [CrossRef]
- Marfels, C.; Hoehn, M.; Wagner, E.; Günther, M. Characterization of in Vivo Chemoresistant Human Hepatocellular Carcinoma Cells with Transendothelial Differentiation Capacities. BMC Cancer 2013, 13, 176. [Google Scholar] [CrossRef]
- Wu, L.Q.; Zhang, W.J.; Niu, J.X.; Ye, L.Y.; Yang, Z.H.; Grau, G.E.; Lou, J.N. Phenotypic and Functional Differences between Human Liver Cancer Endothelial Cells and Liver Sinusoidal Endothelial Cells. J. Vasc. Res. 2008, 45, 78–86. [Google Scholar] [CrossRef]
- Thompson, K.J.; Austin, R.G.; Nazari, S.S.; Gersin, K.S.; Iannitti, D.A.; McKillop, I.H. Altered Fatty Acid-Binding Protein 4 (FABP4) Expression and Function in Human and Animal Models of Hepatocellular Carcinoma. Liver Int. 2018, 38, 1074–1083. [Google Scholar] [CrossRef]
- Laouirem, S.; Sannier, A.; Norkowski, E.; Cauchy, F.; Doblas, S.; Rautou, P.E.; Albuquerque, M.; Garteiser, P.; Sognigbé, L.; Raffenne, J.; et al. Endothelial Fatty Liver Binding Protein 4: A New Targetable Mediator in Hepatocellular Carcinoma Related to Metabolic Syndrome. Oncogene 2019, 38, 3033–3046. [Google Scholar] [CrossRef]
- Wang, M.; Liu, J.; Yan, L.; Wang, J.; Jin, Y.; Zheng, Z.-J. Burden of Liver Cancer Attributable to High Fasting Plasma Glucose: A Global Analysis Based on the Global Burden of Disease Study 2019. J. Nutr. Health Aging 2024, 28, 100261. [Google Scholar] [CrossRef] [PubMed]
- Topel, H.; Bağırsakçı, E.; Yılmaz, Y.; Güneş, A.; Bağcı, G.; Çömez, D.; Kahraman, E.; Korhan, P.; Atabey, N. High Glucose Induced C-Met Activation Promotes Aggressive Phenotype and Regulates Expression of Glucose Metabolism Genes in HCC Cells. Sci. Rep. 2021, 11, 11376. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.C.; Maulik, G.; Christensen, J.; Salgia, R. C-Met: Structure, Functions and Potential for Therapeutic Inhibition. Cancer Metastasis Rev. 2003, 22, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Su, X.; Li, Z.; Deng, L.; Liu, X.; Feng, X.; Peng, J. HGF/c-MET Pathway in Cancer: From Molecular Characterization to Clinical Evidence. Oncogene 2021, 40, 4625–4651. [Google Scholar] [CrossRef]
- Fafalios, A.; Ma, J.; Tan, X.; Stoops, J.; Luo, J.; Defrances, M.C.; Zarnegar, R. A Hepatocyte Growth Factor Receptor (Met)-Insulin Receptor Hybrid Governs Hepatic Glucose Metabolism. Nat. Med. 2011, 17, 1577–1584. [Google Scholar] [CrossRef]
- Inoue, H.; Yokoyama, F.; Kita, Y.; Yoshiji, H.; Tsujimoto, T.; Deguchi, A.; Nakai, S.; Morishita, A.; Uchida, N.; Masaki, T.; et al. Relationship between the Proliferative Capability of Hepatocytes and the Intrahepatic Expression of Hepatocyte Growth Factor and C-Met in the Course of Cirrhosis Development in Rats. Int. J. Mol. Med. 2006, 17, 857–864. [Google Scholar] [CrossRef][Green Version]
- Labib, P.L.; Goodchild, G.; Pereira, S.P. Molecular Pathogenesis of Cholangiocarcinoma. BMC Cancer 2019, 19, 185. [Google Scholar] [CrossRef]
- Huang, X.; Gan, G.; Wang, X.; Xu, T.; Xie, W. The HGF-MET Axis Coordinates Liver Cancer Metabolism and Autophagy for Chemotherapeutic Resistance. Autophagy 2019, 15, 1258–1279. [Google Scholar] [CrossRef]
- Biddinger, S.B.; Haas, J.T.; Yu, B.B.; Bezy, O.; Jing, E.; Zhang, W.; Unterman, T.G.; Carey, M.C.; Kahn, C.R. Hepatic Insulin Resistance Directly Promotes Formation of Cholesterol Gallstones. Nat. Med. 2008, 14, 778–782. [Google Scholar] [CrossRef]
- Portincasa, P.; Khalil, M.; Mahdi, L.; Perniola, V.; Idone, V.; Graziani, A.; Baffy, G.; Di Ciaula, A. Metabolic Dysfunction-Associated Steatotic Liver Disease: From Pathogenesis to Current Therapeutic Options. Int. J. Mol. Sci. 2024, 25, 5640. [Google Scholar] [CrossRef]
- Ralston, J.C.; Lyons, C.L.; Kennedy, E.B.; Kirwan, A.M.; Roche, H.M. Fatty Acids and NLRP3 Inflammasome-Mediated Inflammation in Metabolic Tissues. Annu. Rev. Nutr. 2017, 37, 77–102. [Google Scholar] [CrossRef] [PubMed]
- Nisr, R.B.; Shah, D.S.; Ganley, I.G.; Hundal, H.S. Proinflammatory NFkB Signalling Promotes Mitochondrial Dysfunction in Skeletal Muscle in Response to Cellular Fuel Overloading. Cell. Mol. Life Sci. 2019, 76, 4887–4904. [Google Scholar] [CrossRef] [PubMed]
- Niranjan, S.; Phillips, B.E.; Giannoukakis, N. Uncoupling Hepatic Insulin Resistance—Hepatic Inflammation to Improve Insulin Sensitivity and to Prevent Impaired Metabolism-Associated Fatty Liver Disease in Type 2 Diabetes. Front. Endocrinol. 2023, 14, 1193373. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Kobara, H.; Oura, K.; Masaki, T. Mechanisms Underlying Hepatocellular Carcinoma Progression in Patients with Type 2 Diabetes. J. Hepatocell. Carcinoma 2021, 8, 45–55. [Google Scholar] [CrossRef]
- You, Z.; Bei, L.; Cheng, L.P.; Cheng, N.S. Expression of COX-2 and VEGF-C in Cholangiocarcinomas at Different Clinical and Pathological Stages. Genet. Mol. Res. 2015, 14, 6239–6246. [Google Scholar] [CrossRef]
- Breinig, M.; Schirmacher, P.; Kern, M.A. Cyclooxygenase-2 (COX-2)--a Therapeutic Target in Liver Cancer? Curr. Pharm. Des. 2007, 13, 3305–3315. [Google Scholar] [CrossRef]
- Wang, Q.; Lu, D.; Fan, L.; Li, Y.; Liu, Y.; Yu, H.; Wang, H.; Liu, J.; Sun, G. COX-2 Induces Apoptosis-Resistance in Hepatocellular Carcinoma Cells via the HIF-1α/PKM2 Pathway. Int. J. Mol. Med. 2019, 43, 475–488. [Google Scholar] [CrossRef]
- Mai, Y.; Meng, L.; Deng, G.; Qin, Y. The Role of Type 2 Diabetes Mellitus-Related Risk Factors and Drugs in Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2024, 11, 159–171. [Google Scholar] [CrossRef]
- Zhang, Z.; Lai, G.-H.; Sirica, A.E. Celecoxib-Induced Apoptosis in Rat Cholangiocarcinoma Cells Mediated by Akt Inactivation and Bax Translocation. Hepatology 2004, 39, 1028–1037. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Leng, J.; Demetris, A.J.; Wu, T. Cyclooxygenase-2 Promotes Human Cholangiocarcinoma Growth: Evidence for Cyclooxygenase-2-Independent Mechanism in Celecoxib-Mediated Induction of P21waf1/Cip1 and P27kip1 and Cell Cycle Arrest. Cancer Res. 2004, 64, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Spirlì, C.; Fabris, L.; Duner, E.; Fiorotto, R.; Ballardini, G.; Roskams, T.; Larusso, N.F.; Sonzogni, A.; Okolicsanyi, L.; Strazzabosco, M. Cytokine-Stimulated Nitric Oxide Production Inhibits Adenylyl Cyclase and cAMP-Dependent Secretion in Cholangiocytes. Gastroenterology 2003, 124, 737–753. [Google Scholar] [CrossRef] [PubMed]
- Léveillé, M.; Estall, J.L. Mitochondrial Dysfunction in the Transition from NASH to HCC. Metabolites 2019, 9, 233. [Google Scholar] [CrossRef]
- Alzahrani, B.; Iseli, T.J.; Hebbard, L.W. Non-Viral Causes of Liver Cancer: Does Obesity Led Inflammation Play a Role? Cancer Lett. 2014, 345, 223–229. [Google Scholar] [CrossRef]
- Chang, C.-H.; Chang, Y.-T.; Tseng, T.-H.; Wang, C.-J. Mulberry Leaf Extract Inhibit Hepatocellular Carcinoma Cell Proliferation via Depressing IL-6 and TNF-α Derived from Adipocyte. J. Food Drug Anal. 2018, 26, 1024–1032. [Google Scholar] [CrossRef]
- Kobayashi, S.; Werneburg, N.W.; Bronk, S.F.; Kaufmann, S.H.; Gores, G.J. Interleukin-6 Contributes to Mcl-1 up-Regulation and TRAIL Resistance via an Akt-Signaling Pathway in Cholangiocarcinoma Cells. Gastroenterology 2005, 128, 2054–2065. [Google Scholar] [CrossRef]
- Zabron, A.; Edwards, R.J.; Khan, S.A. The Challenge of Cholangiocarcinoma: Dissecting the Molecular Mechanisms of an Insidious Cancer. Dis. Model. Mech. 2013, 6, 281–292. [Google Scholar] [CrossRef]
- Frampton, G.; Invernizzi, P.; Bernuzzi, F.; Pae, H.Y.; Quinn, M.; Horvat, D.; Galindo, C.; Huang, L.; McMillin, M.; Cooper, B.; et al. Interleukin-6-Driven Progranulin Expression Increases Cholangiocarcinoma Growth by an Akt-Dependent Mechanism. Gut 2012, 61, 268–277. [Google Scholar] [CrossRef]
- Fava, G.; Alpini, G.; Rychlicki, C.; Saccomanno, S.; DeMorrow, S.; Trozzi, L.; Candelaresi, C.; Venter, J.; Di Sario, A.; Marzioni, M.; et al. Leptin Enhances Cholangiocarcinoma Cell Growth. Cancer Res. 2008, 68, 6752–6761. [Google Scholar] [CrossRef]
- Cadamuro, M.; Lasagni, A.; Sarcognato, S.; Guido, M.; Fabris, R.; Strazzabosco, M.; Strain, A.J.; Simioni, P.; Villa, E.; Fabris, L. The Neglected Role of Bile Duct Epithelial Cells in NASH. Semin. Liver Dis. 2022, 42, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Manieri, E.; Folgueira, C.; Rodríguez, M.E.; Leiva-Vega, L.; Esteban-Lafuente, L.; Chen, C.; Cubero, F.J.; Barrett, T.; Cavanagh-Kyros, J.; Seruggia, D.; et al. JNK-Mediated Disruption of Bile Acid Homeostasis Promotes Intrahepatic Cholangiocarcinoma. Proc. Natl. Acad. Sci. USA 2020, 117, 16492–16499. [Google Scholar] [CrossRef]
- Romeo, M.; Dallio, M.; Di Nardo, F.; Napolitano, C.; Vaia, P.; Martinelli, G.; Federico, P.; Olivieri, S.; Iodice, P.; Federico, A. The Role of the Gut-Biliary-Liver Axis in Primary Hepatobiliary Liver Cancers: From Molecular Insights to Clinical Applications. J. Pers. Med. 2025, 15, 124. [Google Scholar] [CrossRef]
- Gravina, A.G.; Romeo, M.; Pellegrino, R.; Tuccillo, C.; Federico, A.; Loguercio, C. Just Drink a Glass of Water? Effects of Bicarbonate-Sulfate-Calcium-Magnesium Water on the Gut-Liver Axis. Front. Pharmacol. 2022, 13, 869446. [Google Scholar] [CrossRef]
- Dallio, M.; Romeo, M.; Gravina, A.G.; Masarone, M.; Larussa, T.; Abenavoli, L.; Persico, M.; Loguercio, C.; Federico, A. Nutrigenomics and Nutrigenetics in Metabolic- (Dysfunction) Associated Fatty Liver Disease: Novel Insights and Future Perspectives. Nutrients 2021, 13, 1679. [Google Scholar] [CrossRef]
- Federico, A.; Dallio, M.; Caprio, G.G.; Ormando, V.M.; Loguercio, C. Gut Microbiota and the Liver. Minerva Gastroenterol. Dietol. 2017, 63, 385–398. [Google Scholar] [CrossRef]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.-M.; Kennedy, S.; et al. Richness of Human Gut Microbiome Correlates with Metabolic Markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Larsen, N.; Vogensen, F.K.; van den Berg, F.W.J.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut Microbiota in Human Adults with Type 2 Diabetes Differs from Non-Diabetic Adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef]
- Bielka, W.; Przezak, A.; Pawlik, A. The Role of the Gut Microbiota in the Pathogenesis of Diabetes. Int. J. Mol. Sci. 2022, 23, 480. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.H.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human Gut Microbes Impact Host Serum Metabolome and Insulin Sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Benedé-Ubieto, R.; Cubero, F.J.; Nevzorova, Y.A. Breaking the Barriers: The Role of Gut Homeostasis in Metabolic-Associated Steatotic Liver Disease (MASLD). Gut Microbes 2024, 16, 2331460. [Google Scholar] [CrossRef]
- Ju, T.; Bourrie, B.C.T.; Forgie, A.J.; Pepin, D.M.; Tollenaar, S.; Sergi, C.M.; Willing, B.P. The Gut Commensal Escherichia coli Aggravates High-Fat-Diet-Induced Obesity and Insulin Resistance in Mice. Appl. Environ. Microbiol. 2023, 89, e0162822. [Google Scholar] [CrossRef] [PubMed]
- Grąt, M.; Wronka, K.M.; Krasnodębski, M.; Masior, Ł.; Lewandowski, Z.; Kosińska, I.; Grąt, K.; Stypułkowski, J.; Rejowski, S.; Wasilewicz, M.; et al. Profile of Gut Microbiota Associated with the Presence of Hepatocellular Cancer in Patients with Liver Cirrhosis. Transplant. Proc. 2016, 48, 1687–1691. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, R.F.; Seki, E.; Brenner, D.A. Toll-like Receptor Signaling in the Liver. Gastroenterology 2006, 130, 1886–1900. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Guo, S.; Zhou, Y.; Zhao, J.; Wang, M.; Sang, L.; Chang, B.; Wang, B. Hepatocellular Carcinoma: How the Gut Microbiota Contributes to Pathogenesis, Diagnosis, and Therapy. Front. Microbiol. 2022, 13, 873160. [Google Scholar] [CrossRef]
- Dapito, D.H.; Mencin, A.; Gwak, G.-Y.; Pradere, J.-P.; Jang, M.-K.; Mederacke, I.; Caviglia, J.M.; Khiabanian, H.; Adeyemi, A.; Bataller, R.; et al. Promotion of Hepatocellular Carcinoma by the Intestinal Microbiota and TLR4. Cancer Cell 2012, 21, 504–516. [Google Scholar] [CrossRef]
- Sameni, F.; Elkhichi, P.A.; Dadashi, A.; Sadeghi, M.; Goudarzi, M.; Eshkalak, M.P.; Dadashi, M. Global Prevalence of Fusobacterium Nucleatum and Bacteroides Fragilis in Patients with Colorectal Cancer: An Overview of Case Reports/Case Series and Meta-Analysis of Prevalence Studies. BMC Gastroenterol. 2025, 25, 71. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Cui, J.; Zhu, M.; Wang, W.; Chen, K.; Huang, L.; Liu, Y. Oral-Gut Microbial Transmission Promotes Diabetic Coronary Heart Disease. Cardiovasc. Diabetol. 2024, 23, 123. [Google Scholar] [CrossRef]
- Bullman, S.; Pedamallu, C.S.; Sicinska, E.; Clancy, T.E.; Zhang, X.; Cai, D.; Neuberg, D.; Huang, K.; Guevara, F.; Nelson, T.; et al. Analysis of Fusobacterium Persistence and Antibiotic Response in Colorectal Cancer. Science 2017, 358, 1443–1448. [Google Scholar] [CrossRef]
- Shi, G.; Lin, Y.; Wu, Y.; Zhou, J.; Cao, L.; Chen, J.; Li, Y.; Tan, N.; Zhong, S. Bacteroides Fragilis Supplementation Deteriorated Metabolic Dysfunction, Inflammation, and Aorta Atherosclerosis by Inducing Gut Microbiota Dysbiosis in Animal Model. Nutrients 2022, 14, 2199. [Google Scholar] [CrossRef]
- Yoshimoto, S.; Loo, T.M.; Atarashi, K.; Kanda, H.; Sato, S.; Oyadomari, S.; Iwakura, Y.; Oshima, K.; Morita, H.; Hattori, M.; et al. Obesity-Induced Gut Microbial Metabolite Promotes Liver Cancer through Senescence Secretome. Nature 2013, 499, 97–101. [Google Scholar] [CrossRef]
- Wang, X.; Fang, Y.; Liang, W.; Cai, Y.; Wong, C.C.; Wang, J.; Wang, N.; Lau, H.C.-H.; Jiao, Y.; Zhou, X.; et al. Gut-Liver Translocation of Pathogen Klebsiella Pneumoniae Promotes Hepatocellular Carcinoma in Mice. Nat. Microbiol. 2025, 10, 169–184. [Google Scholar] [CrossRef]
- Herraez, E.; Romero, M.R.; Macias, R.I.R.; Monte, M.J.; Marin, J.J.G. Clinical Relevance of the Relationship between Changes in Gut Microbiota and Bile Acid Metabolism in Patients with Intrahepatic Cholangiocarcinoma. Hepatobiliary Surg. Nutr. 2020, 9, 211–214. [Google Scholar] [CrossRef]
- Swann, J.R.; Want, E.J.; Geier, F.M.; Spagou, K.; Wilson, I.D.; Sidaway, J.E.; Nicholson, J.K.; Holmes, E. Systemic Gut Microbial Modulation of Bile Acid Metabolism in Host Tissue Compartments. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4523–4530. [Google Scholar] [CrossRef]
- Sinha, S.R.; Haileselassie, Y.; Nguyen, L.P.; Tropini, C.; Wang, M.; Becker, L.S.; Sim, D.; Jarr, K.; Spear, E.T.; Singh, G.; et al. Dysbiosis-Induced Secondary Bile Acid Deficiency Promotes Intestinal Inflammation. Cell Host Microbe 2020, 27, 659–670.e5. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B.; Bajaj, J.S. Bile Acids and the Gut Microbiome. Curr. Opin. Gastroenterol. 2014, 30, 332–338. [Google Scholar] [CrossRef]
- Sang, H.; Li, W.J.; Zhou, Y.; Zhu, H. Advances in research on the relationship between bile acid, gut microbiota and the occurrence and development of cholangiocarcinoma. Zhonghua Gan Zang Bing Za Zhi 2021, 29, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Dejea, C.M.; Fathi, P.; Craig, J.M.; Boleij, A.; Taddese, R.; Geis, A.L.; Wu, X.; DeStefano Shields, C.E.; Hechenbleikner, E.M.; Huso, D.L.; et al. Patients with Familial Adenomatous Polyposis Harbor Colonic Biofilms Containing Tumorigenic Bacteria. Science 2018, 359, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Ridlon, J.M.; Kang, D.-J.; Hylemon, P.B. Bile Salt Biotransformations by Human Intestinal Bacteria. J. Lipid Res. 2006, 47, 241–259. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.G.; Feng, Y.; Theve, E.J.; Raczynski, A.R.; Fiala, J.L.A.; Doernte, A.L.; Williams, M.; McFaline, J.L.; Essigmann, J.M.; Schauer, D.B.; et al. Gut Microbes Define Liver Cancer Risk in Mice Exposed to Chemical and Viral Transgenic Hepatocarcinogens. Gut 2010, 59, 88–97. [Google Scholar] [CrossRef]
- Gros, B.; Gómez Pérez, A.; Pleguezuelo, M.; Serrano Ruiz, F.J.; de la Mata, M.; Rodríguez-Perálvarez, M. Helicobacter Species and Hepato-Biliary Tract Malignancies: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 595. [Google Scholar] [CrossRef]
- Jia, W.; Xie, G.; Jia, W. Bile Acid-Microbiota Crosstalk in Gastrointestinal Inflammation and Carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 111–128. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Wolf, P.G.; Gaskins, H.R. Taurocholic Acid Metabolism by Gut Microbes and Colon Cancer. Gut Microbes 2016, 7, 201–215. [Google Scholar] [CrossRef]
- Wirbel, J.; Pyl, P.T.; Kartal, E.; Zych, K.; Kashani, A.; Milanese, A.; Fleck, J.S.; Voigt, A.Y.; Palleja, A.; Ponnudurai, R.; et al. Meta-Analysis of Fecal Metagenomes Reveals Global Microbial Signatures That Are Specific for Colorectal Cancer. Nat. Med. 2019, 25, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Hall, M.N. Metabolic Reprogramming in Hepatocellular Carcinoma: Mechanisms and Therapeutic Implications. Exp. Mol. Med. 2025, 57, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, J.; Lin, H.; Lin, B.; Zhu, M.; Li, M. Glucose Metabolism Reprogramming Promotes Immune Escape of Hepatocellular Carcinoma Cells. Explor. Target. Antitumor Ther. 2023, 4, 519–536. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Shime, H.; Yabu, M.; Akazawa, T.; Kodama, K.; Matsumoto, M.; Seya, T.; Inoue, N. Tumor-Secreted Lactic Acid Promotes IL-23/IL-17 Proinflammatory Pathway. J. Immunol. 2008, 180, 7175–7183. [Google Scholar] [CrossRef]
- Xia, L.; Oyang, L.; Lin, J.; Tan, S.; Han, Y.; Wu, N.; Yi, P.; Tang, L.; Pan, Q.; Rao, S.; et al. The Cancer Metabolic Reprogramming and Immune Response. Mol. Cancer 2021, 20, 28. [Google Scholar] [CrossRef]
- Chang, C.-H.; Qiu, J.; O’Sullivan, D.; Buck, M.D.; Noguchi, T.; Curtis, J.D.; Chen, Q.; Gindin, M.; Gubin, M.M.; van der Windt, G.J.W.; et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell 2015, 162, 1229–1241. [Google Scholar] [CrossRef]
- Brand, A.; Singer, K.; Koehl, G.E.; Kolitzus, M.; Schoenhammer, G.; Thiel, A.; Matos, C.; Bruss, C.; Klobuch, S.; Peter, K.; et al. LDHA-Associated Lactic Acid Production Blunts Tumor Immunosurveillance by T and NK Cells. Cell Metab. 2016, 24, 657–671. [Google Scholar] [CrossRef]
- Maciver, N.J.; Jacobs, S.R.; Wieman, H.L.; Wofford, J.A.; Coloff, J.L.; Rathmell, J.C. Glucose Metabolism in Lymphocytes Is a Regulated Process with Significant Effects on Immune Cell Function and Survival. J. Leukoc. Biol. 2008, 84, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Yamada, A.; Arakaki, R.; Saito, M.; Kudo, Y.; Ishimaru, N. Dual Role of Fas/FasL-Mediated Signal in Peripheral Immune Tolerance. Front. Immunol. 2017, 8, 403. [Google Scholar] [CrossRef]
- Lu, M.; Wu, Y.; Xia, M.; Zhang, Y. The Role of Metabolic Reprogramming in Liver Cancer and Its Clinical Perspectives. Front. Oncol. 2024, 14, 1454161. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, H. Heterogeneity of Liver Cancer and Personalized Therapy. Cancer Lett. 2016, 379, 191–197. [Google Scholar] [CrossRef]
- Wang, W.-M.; Xu, Y.; Yang, X.-R.; Wang, Y.-H.; Sun, H.-X.; Fan, J. Prognostic Role of Diabetes Mellitus in Hepatocellular Carcinoma Patients after Curative Treatments: A Meta-Analysis. Hepatobiliary Pancreat. Dis. Int. 2011, 10, 346–355. [Google Scholar] [CrossRef]
- Leyh, C.; Coombes, J.D.; Schmidt, H.H.; Canbay, A.; Manka, P.P.; Best, J. MASLD-Related HCC-Update on Pathogenesis and Current Treatment Options. J. Pers. Med. 2024, 14, 370. [Google Scholar] [CrossRef]
- Pernicova, I.; Korbonits, M. Metformin--Mode of Action and Clinical Implications for Diabetes and Cancer. Nat. Rev. Endocrinol. 2014, 10, 143–156. [Google Scholar] [CrossRef]
- Wu, H.; Esteve, E.; Tremaroli, V.; Khan, M.T.; Caesar, R.; Mannerås-Holm, L.; Ståhlman, M.; Olsson, L.M.; Serino, M.; Planas-Fèlix, M.; et al. Metformin Alters the Gut Microbiome of Individuals with Treatment-Naive Type 2 Diabetes, Contributing to the Therapeutic Effects of the Drug. Nat. Med. 2017, 23, 850–858. [Google Scholar] [CrossRef]
- Sun, L.; Xie, C.; Wang, G.; Wu, Y.; Wu, Q.; Wang, X.; Liu, J.; Deng, Y.; Xia, J.; Chen, B.; et al. Gut Microbiota and Intestinal FXR Mediate the Clinical Benefits of Metformin. Nat. Med. 2018, 24, 1919–1929. [Google Scholar] [CrossRef]
- de la Cuesta-Zuluaga, J.; Mueller, N.T.; Corrales-Agudelo, V.; Velásquez-Mejía, E.P.; Carmona, J.A.; Abad, J.M.; Escobar, J.S. Metformin Is Associated With Higher Relative Abundance of Mucin-Degrading Akkermansia Muciniphila and Several Short-Chain Fatty Acid-Producing Microbiota in the Gut. Diabetes Care 2017, 40, 54–62. [Google Scholar] [CrossRef]
- Zhou, J.; Ke, Y.; Lei, X.; Wu, T.; Li, Y.; Bao, T.; Tang, H.; Zhang, C.; Wu, X.; Wang, G.; et al. Meta-Analysis: The Efficacy of Metformin and Other Anti-Hyperglycemic Agents in Prolonging the Survival of Hepatocellular Carcinoma Patients with Type 2 Diabetes. Ann. Hepatol. 2020, 19, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.M. SGLT2 Inhibitors: Physiology and Pharmacology. Kidney360 2021, 2, 2027–2037. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.; Heerspink, H.J.L.; Cuthbertson, D.J.; Wilding, J.P.H. SGLT2 Inhibitors and GLP-1 Receptor Agonists: Established and Emerging Indications. Lancet 2021, 398, 262–276. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Xu, Y.; Wang, D.; Chen, F.; Tu, Z.; Qian, J.; Xu, S.; Xu, Y.; Hwa, J.; Li, J.; et al. Cardioprotective Mechanism of SGLT2 Inhibitor against Myocardial Infarction Is through Reduction of Autosis. Protein Cell 2022, 13, 336–359. [Google Scholar] [CrossRef] [PubMed]
- Amjad, W.; Malik, A.; Qureshi, W.; Dennis, B.; Mumtaz, M.; Haider, R.; Jamal, S.; Jaura, F.; Ahmed, A. Sodium-Glucose Cotransporter-2 Inhibitors Improve Liver Enzymes in Patients with Co-Existing Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Prz. Gastroenterol. 2022, 17, 288–300. [Google Scholar] [CrossRef]
- Taguchi, D.; Shirakami, Y.; Sakai, H.; Minowa, D.; Miwa, T.; Maeda, T.; Kubota, M.; Imai, K.; Ibuka, T.; Shimizu, M. Dual Roles of Canagliflozin on Cholangiocarcinoma Cell Growth and Enhanced Growth Suppression in Combination with FK866. Int. J. Mol. Sci. 2025, 26, 978. [Google Scholar] [CrossRef]
- Ong Lopez, A.M.C.; Pajimna, J.A.T. Efficacy of Sodium Glucose Cotransporter 2 Inhibitors on Hepatic Fibrosis and Steatosis in Non-Alcoholic Fatty Liver Disease: An Updated Systematic Review and Meta-Analysis. Sci. Rep. 2024, 14, 2122. [Google Scholar] [CrossRef]
- Huynh, D.J.; Renelus, B.D.; Jamorabo, D.S. Reduced Mortality and Morbidity Associated with Metformin and SGLT2 Inhibitor Therapy in Patients with Type 2 Diabetes Mellitus and Cirrhosis. BMC Gastroenterol. 2023, 23, 450. [Google Scholar] [CrossRef]
- Scheen, A.J. An Update on the Safety of SGLT2 Inhibitors. Expert. Opin. Drug Saf. 2019, 18, 295–311. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, J.-H.; Jung, E.-J.; Park, W.; Seo, J.; Kang, M.; Jung, E.H.; Kim, S.-A.; Suh, K.J.; Kim, J.-W.; et al. Prevalence and Thrombotic Risk of SGLT-2 Inhibitor-Associated Erythrocytosis: A Retrospective Cohort Study. Cardiovasc. Diabetol. 2025, 24, 276. [Google Scholar] [CrossRef]
- Nishina, S.; Hino, K. CD26/DPP4 as a Therapeutic Target in Nonalcoholic Steatohepatitis Associated Hepatocellular Carcinoma. Cancers 2022, 14, 454. [Google Scholar] [CrossRef] [PubMed]
- Eitah, H.; Sayed, R.; Maklad, Y.; Gamal el Din, A.; Mahmoud, K.; Elsahar, A.; Alhejely, A.; Abdulbaqi, A.; Naeim, H. Dipeptidyl Peptidase-4 Enzyme Inhibition and Its Impacts on Hepatic Preneoplasia: A New Avenue for Liver Cancer Management. Front. Pharmacol. 2025, 16, 1559303. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Nakano, D.; Koga, H.; Torimura, T. Effects of a DPP4 Inhibitor on Progression of NASH-Related HCC and the P62/ Keap1/Nrf2-Pentose Phosphate Pathway in a Mouse Model. Liver Cancer 2019, 8, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Chou, O.H.I.; Ning, J.; Chan, R.N.C.; Chung, C.T.; Huang, H.; Ng, K.; Dee, E.C.; Lee, S.; Kaewdech, A.; Chow, A.K.M.; et al. Lower Risks of New-Onset Hepatocellular Carcinoma in Patients With Type 2 Diabetes Mellitus Treated With SGLT2 Inhibitors Versus DPP4 Inhibitors. J. Natl. Compr. Cancer Netw. 2024, 22, e237118. [Google Scholar] [CrossRef]
- Dicembrini, I.; Nreu, B.; Montereggi, C.; Mannucci, E.; Monami, M. Risk of Cancer in Patients Treated with Dipeptidyl Peptidase-4 Inhibitors: An Extensive Meta-Analysis of Randomized Controlled Trials. Acta Diabetol. 2020, 57, 689–696. [Google Scholar] [CrossRef]
- Oh, Y.S.; Jun, H.-S. Effects of Glucagon-Like Peptide-1 on Oxidative Stress and Nrf2 Signaling. Int. J. Mol. Sci. 2017, 19, 26. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Gaunt, P.; Aithal, G.P.; Barton, D.; Hull, D.; Parker, R.; Hazlehurst, J.M.; Guo, K.; LEAN trial team; Abouda, G.; et al. Liraglutide Safety and Efficacy in Patients with Non-Alcoholic Steatohepatitis (LEAN): A Multicentre, Double-Blind, Randomised, Placebo-Controlled Phase 2 Study. Lancet 2016, 387, 679–690. [Google Scholar] [CrossRef]
- Kanwal, F.; Kramer, J.R.; Li, L.; Yang, Y.-X.; Cao, Y.; Yu, X.; Samuel, R.; Ali, B.; Desiderio, R.; Cholankeril, G.; et al. GLP-1 Receptor Agonists and Risk for Cirrhosis and Related Complications in Patients With Metabolic Dysfunction-Associated Steatotic Liver Disease. JAMA Intern. Med. 2024, 184, 1314–1323. [Google Scholar] [CrossRef]
- Wang, L.; Berger, N.A.; Kaelber, D.C.; Xu, R. Association of GLP-1 Receptor Agonists and Hepatocellular Carcinoma Incidence and Hepatic Decompensation in Patients With Type 2 Diabetes. Gastroenterology 2024, 167, 689–703. [Google Scholar] [CrossRef]
- Xie, Y.; Choi, T.; Al-Aly, Z. Mapping the Effectiveness and Risks of GLP-1 Receptor Agonists. Nat. Med. 2025, 31, 951–962. [Google Scholar] [CrossRef]
- Filippatos, T.D.; Panagiotopoulou, T.V.; Elisaf, M.S. Adverse Effects of GLP-1 Receptor Agonists. Rev. Diabet. Stud. 2014, 11, 202–230. [Google Scholar] [CrossRef]
- Bezin, J.; Gouverneur, A.; Pénichon, M.; Mathieu, C.; Garrel, R.; Hillaire-Buys, D.; Pariente, A.; Faillie, J.-L. GLP-1 Receptor Agonists and the Risk of Thyroid Cancer. Diabetes Care 2023, 46, 384–390. [Google Scholar] [CrossRef]
- Brito, J.P.; Herrin, J.; Swarna, K.S.; Singh Ospina, N.M.; Montori, V.M.; Toro-Tobon, D.; Umpierrez, G.E.; Galindo, R.J.; Deng, Y.; Mickelson, M.M.; et al. GLP-1RA Use and Thyroid Cancer Risk. JAMA Otolaryngol. Head. Neck Surg. 2025, 151, 243. [Google Scholar] [CrossRef] [PubMed]
- Baxter, S.M.; Lund, L.C.; Andersen, J.H.; Brix, T.H.; Hegedüs, L.; Hsieh, M.H.-C.; Su, C.T.-T.; Cheng, M.C.-Y.; Chang, Z.C.-J.; Lai, E.C.-C.; et al. Glucagon-Like Peptide 1 Receptor Agonists and Risk of Thyroid Cancer: An International Multisite Cohort Study. Thyroid 2025, 35, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Armandi, A.; Bugianesi, E. Dietary and Pharmacological Treatment in Patients with Metabolic-Dysfunction Associated Steatotic Liver Disease. Eur. J. Intern. Med. 2024, 122, 20–27. [Google Scholar] [CrossRef]
- Romeo, M.; Dallio, M.; Napolitano, C.; Basile, C.; Di Nardo, F.; Vaia, P.; Iodice, P.; Federico, A. Clinical Applications of Artificial Intelligence (AI) in Human Cancer: Is It Time to Update the Diagnostic and Predictive Models in Managing Hepatocellular Carcinoma (HCC)? Diagnostics 2025, 15, 252. [Google Scholar] [CrossRef]
- McTeer, M.; Applegate, D.; Mesenbrink, P.; Ratziu, V.; Schattenberg, J.M.; Bugianesi, E.; Geier, A.; Romero Gomez, M.; Dufour, J.-F.; Ekstedt, M.; et al. Machine Learning Approaches to Enhance Diagnosis and Staging of Patients with MASLD Using Routinely Available Clinical Information. PLoS ONE 2024, 19, e0299487. [Google Scholar] [CrossRef]
- Mortazavi, B.J.; Gutierrez-Osuna, R. A Review of Digital Innovations for Diet Monitoring and Precision Nutrition. J. Diabetes Sci. Technol. 2023, 17, 217–223. [Google Scholar] [CrossRef]
- Shea, B.; Bakre, S.; Carano, K.; Scharen, J.; Langheier, J.; Hu, E.A. Changes in Glycemic Control Among Individuals With Diabetes Who Used a Personalized Digital Nutrition Platform: Longitudinal Study. JMIR Diabetes 2021, 6, e32298. [Google Scholar] [CrossRef]
- Hu, E.A.; Scharen, J.; Nguyen, V.; Langheier, J. Evaluating the Impact of a Digital Nutrition Platform on Cholesterol Levels in Users With Dyslipidemia: Longitudinal Study. JMIR Cardio 2021, 5, e28392. [Google Scholar] [CrossRef]
- Qin, H.; Yuan, B.; Huang, W.; Wang, Y. Utilizing Gut Microbiota to Improve Hepatobiliary Tumor Treatments: Recent Advances. Front. Oncol. 2022, 12, 924696. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Yang, C.; Ren, K.; Xu, M.; Pan, C.; Ye, X.; Li, L. Modulation of Gut Microbiota by Probiotics to Improve the Efficacy of Immunotherapy in Hepatocellular Carcinoma. Front. Immunol. 2024, 15, 1504948. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Cai, C.; Wang, C.; Chen, X.; Zhang, B.; Huang, Z. Gut Microbiota-Mediated Gut-Liver Axis: A Breakthrough Point for Understanding and Treating Liver Cancer. Clin. Mol. Hepatol. 2025, 31, 350–381. [Google Scholar] [CrossRef] [PubMed]
- Ting, N.L.-N.; Lau, H.C.-H.; Yu, J. Cancer Pharmacomicrobiomics: Targeting Microbiota to Optimise Cancer Therapy Outcomes. Gut 2022, 71, 1412–1425. [Google Scholar] [CrossRef]
- Fulgenzi, C.A.M.; Cheon, J.; D’Alessio, A.; Nishida, N.; Ang, C.; Marron, T.U.; Wu, L.; Saeed, A.; Wietharn, B.; Cammarota, A.; et al. Reproducible Safety and Efficacy of Atezolizumab plus Bevacizumab for HCC in Clinical Practice: Results of the AB-Real Study. Eur. J. Cancer 2022, 175, 204–213. [Google Scholar] [CrossRef]
- Kelley, R.K.; Bridgewater, J.; Gores, G.J.; Zhu, A.X. Systemic Therapies for Intrahepatic Cholangiocarcinoma. J. Hepatol. 2020, 72, 353–363. [Google Scholar] [CrossRef]
- Bekkering, S.; Domínguez-Andrés, J.; Joosten, L.A.B.; Riksen, N.P.; Netea, M.G. Trained Immunity: Reprogramming Innate Immunity in Health and Disease. Annu. Rev. Immunol. 2021, 39, 667–693. [Google Scholar] [CrossRef]
- Netea, M.G.; Domínguez-Andrés, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.B.; van der Meer, J.W.M.; Mhlanga, M.M.; Mulder, W.J.M.; et al. Defining Trained Immunity and Its Role in Health and Disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [CrossRef]
- Hsieh, C.-H.; Chuang, P.-C.; Liu, Y.-W. Beyond Adaptive Immunity: Trained Innate Immune Responses as a Novel Frontier in Hepatocellular Carcinoma Therapy. Cancers 2025, 17, 1250. [Google Scholar] [CrossRef]
- Ochando, J.; Mulder, W.J.M.; Madsen, J.C.; Netea, M.G.; Duivenvoorden, R. Trained Immunity—Basic Concepts and Contributions to Immunopathology. Nat. Rev. Nephrol. 2023, 19, 23–37. [Google Scholar] [CrossRef]
- Sui, Y.; Berzofsky, J.A. Trained Immunity Inducers in Cancer Immunotherapy. Front. Immunol. 2024, 15, 1427443. [Google Scholar] [CrossRef]
- Vaziri, F.; Setayesh, T.; Hu, Y.; Ravindran, R.; Wei, D.; Wan, Y.-J.Y. BCG as an Innovative Option for HCC Treatment: Repurposing and Mechanistic Insights. Adv Sci 2024, 11, e2308242. [Google Scholar] [CrossRef]
- Yarchoan, M.; Gane, E.J.; Marron, T.U.; Perales-Linares, R.; Yan, J.; Cooch, N.; Shu, D.H.; Fertig, E.J.; Kagohara, L.T.; Bartha, G.; et al. Personalized Neoantigen Vaccine and Pembrolizumab in Advanced Hepatocellular Carcinoma: A Phase 1/2 Trial. Nat. Med. 2024, 30, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Mulder, W.J.M.; Ochando, J.; Joosten, L.A.B.; Fayad, Z.A.; Netea, M.G. Therapeutic Targeting of Trained Immunity. Nat. Rev. Drug Discov. 2019, 18, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Szablewski, L. Insulin Resistance: The Increased Risk of Cancers. Curr. Oncol. 2024, 31, 998–1027. [Google Scholar] [CrossRef] [PubMed]
- Kounatidis, D.; Vallianou, N.G.; Karampela, I.; Rebelos, E.; Kouveletsou, M.; Dalopoulos, V.; Koufopoulos, P.; Diakoumopoulou, E.; Tentolouris, N.; Dalamaga, M. Anti-Diabetic Therapies and Cancer: From Bench to Bedside. Biomolecules 2024, 14, 1479. [Google Scholar] [CrossRef]
- Dhas, Y.; Biswas, N.; MR, D.; Jones, L.D.; Ashili, S. Repurposing Metabolic Regulators: Antidiabetic Drugs as Anticancer Agents. Mol. Biomed. 2024, 5, 40. [Google Scholar] [CrossRef]
- Yang, J.; Kim, G.; Shim, J.H.; An, J. Second-Line Antidiabetic Drugs: Friend or Foe of the Liver. J. Liver Cancer 2025, accepted. [Google Scholar] [CrossRef]
- Dang, Q.; Li, B.; Jin, B.; Ye, Z.; Lou, X.; Wang, T.; Wang, Y.; Pan, X.; Hu, Q.; Li, Z.; et al. Cancer Immunometabolism: Advent, Challenges, and Perspective. Mol. Cancer 2024, 23, 72. [Google Scholar] [CrossRef]
- Caini, P.; Carloni, V. Metabolism and Immune Suppressive Response in Liver Cancer. Biomedicines 2025, 13, 1461. [Google Scholar] [CrossRef]
| Bacterial Species | Key Characteristics | Role in HCC Pathogenesis | References |
|---|---|---|---|
| Escherichia coli | Gram-negative; enriched in MD-related gut dysbiosis | Activates TLR4 signaling via LPS; promotes chronic hepatic inflammation and oxidative stress | [107] |
| Klebsiella pneumoniae | Gram-negative; enriched in MD-related gut dysbiosis | Promoting bacterial translocation; activates Kupffer cells and hepatic immune response via LPS | [113] |
| Bacteroides fragilis | Commensal; enriched in MD-related gut dysbiosis contributing to the deterioration of glucose metabolism | Activates TLR4 signaling via LPS; induction of senescence-associated secretory phenotypes in hepatic stellate cells; promotion of chronic hepatic inflammation | [112] |
| Fusobacterium nucleatum | Anaerobic opportunist; associated with T2DM and various GI malignancies | Promotes inflammation and may activate TLR4-dependent oncogenic pathways in tumor-prone environments. | [110] |
| Bacterial Species | Key Characteristics | Role in CCA Pathogenesis | References |
|---|---|---|---|
| Escherichia coli | Gram-negative facultative anaerobe; enriched in MD-related gut dysbiosis | Promotes chronic inflammation and oxidative stress in biliary epithelium via LPS | [119] |
| Clostridium spp. | Anaerobic Firmicutes; capable of 7α-dehydroxylation of primary bile acids; enriched in MD-related gut dysbiosis | Production of secondary bile acids (e.g., deoxycholic acid, DCA) with pro-carcinogenic properties | [112,120] |
| Helicobacter spp. | Bile-resistant; colonizes biliary tract; includes H. hepaticus and H. bilis; enriched in MD-related gut dysbiosis | Chronic inflammation and DNA damage; potential carcinogenic role | [121,122] |
| Bacteroides spp. | Dominant gut anaerobes; key in bile acid and carbohydrate metabolism; enriched in MD-related gut dysbiosis | Reduced abundance disrupts bile acid homeostasis and favors dysbiosis | [123,124] |
| Veillonella spp. | Gram-negative anaerobes; lactate fermenters; enriched in MD-related gut dysbiosis | Promoting local inflammation via LPS/TLR4-dependent pathways | [125] |
| Drug Class | Mechanisms of Action in Hepatic Cancerogenesis | Potential Therapeutic Implications (Type of Supporting Evidence) | Ref. |
|---|---|---|---|
| Metformin | Improving IR and IR-related effects; modulation of gut-liver axis (regulates BAs metabolism) | HCC: reduction of HCC risk and mortality in T2DM patients (metanalysis) CCA: reduction of malignant cells proliferation and invasion potential (preclinical models) | [143] [36] |
| SGLT2-Is | Blocking renal glucose reabsorption thus reducing glucose levels and relative pro-cancerogenic effects; activates NAD+ salvage pathways. | HCC: combination with metformin reduces incidence (retrospective in human evidence) CCA: high doses suppress CCA cell viability (dose-dependent effects in vitro). | [148] [150] |
| DPP4-Is | Inhibition of DPP4/CD26-regulated pathways; modulation of immune response and oxidative stress | HCC: Promotion of apoptosis in HCC cells via suppressing of p62/Keap1/Nrf2 axis (in vitro); reduction of HCC incidence in T2DM-Hepatitis C Virus (HCV) infected patients (in human) CCA: lack of solid evidence | [155] [156] |
| GLP-1 RAs | Enhancing insulin secretion and reducing glucagon levels; promotion of anti-inflammatory effects | HCC: Reduction of HCC incidence in T2DM patients (emerging in vivo evidence) CCA: Limited role in tumor suppression (in vitro & in vivo evidence) | [161] [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romeo, M.; Di Nardo, F.; Napolitano, C.; Basile, C.; Palma, C.; Vaia, P.; Dallio, M.; Federico, A. Exploring the Epidemiologic Burden, Pathogenetic Features, and Clinical Outcomes of Primary Liver Cancer in Patients with Type 2 Diabetes Mellitus (T2DM) and Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A Scoping Review. Diabetology 2025, 6, 79. https://doi.org/10.3390/diabetology6080079
Romeo M, Di Nardo F, Napolitano C, Basile C, Palma C, Vaia P, Dallio M, Federico A. Exploring the Epidemiologic Burden, Pathogenetic Features, and Clinical Outcomes of Primary Liver Cancer in Patients with Type 2 Diabetes Mellitus (T2DM) and Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A Scoping Review. Diabetology. 2025; 6(8):79. https://doi.org/10.3390/diabetology6080079
Chicago/Turabian StyleRomeo, Mario, Fiammetta Di Nardo, Carmine Napolitano, Claudio Basile, Carlo Palma, Paolo Vaia, Marcello Dallio, and Alessandro Federico. 2025. "Exploring the Epidemiologic Burden, Pathogenetic Features, and Clinical Outcomes of Primary Liver Cancer in Patients with Type 2 Diabetes Mellitus (T2DM) and Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A Scoping Review" Diabetology 6, no. 8: 79. https://doi.org/10.3390/diabetology6080079
APA StyleRomeo, M., Di Nardo, F., Napolitano, C., Basile, C., Palma, C., Vaia, P., Dallio, M., & Federico, A. (2025). Exploring the Epidemiologic Burden, Pathogenetic Features, and Clinical Outcomes of Primary Liver Cancer in Patients with Type 2 Diabetes Mellitus (T2DM) and Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A Scoping Review. Diabetology, 6(8), 79. https://doi.org/10.3390/diabetology6080079







