Diabetes Worsens Outcomes After Asphyxial Cardiac Arrest in Rats
Abstract
1. Background
2. Methods
2.1. Diabetes Induction
2.2. Surgical Preparation
2.3. Cardiac Arrest
2.4. Statistics
3. Results
3.1. Diabetic Markers
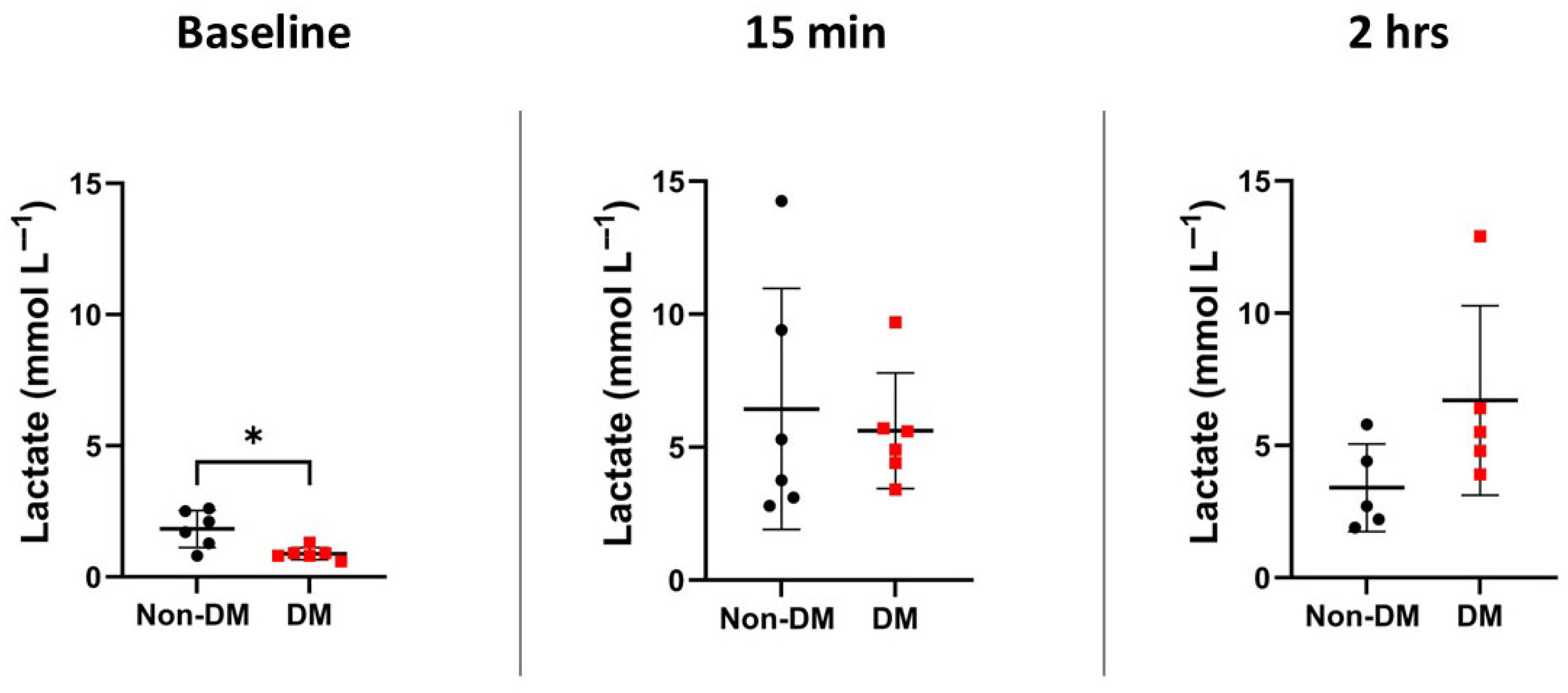
3.2. Markers of Arrest and Recovery
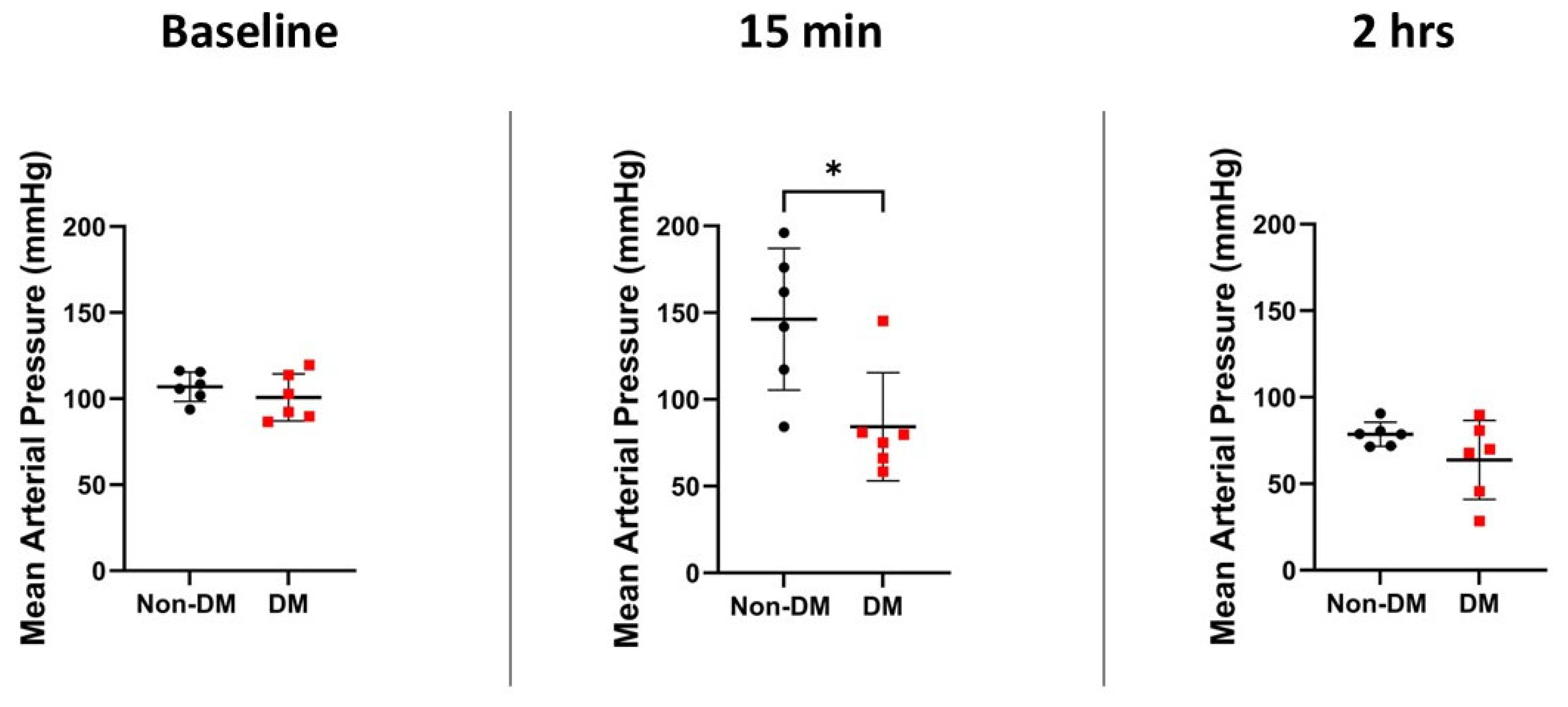
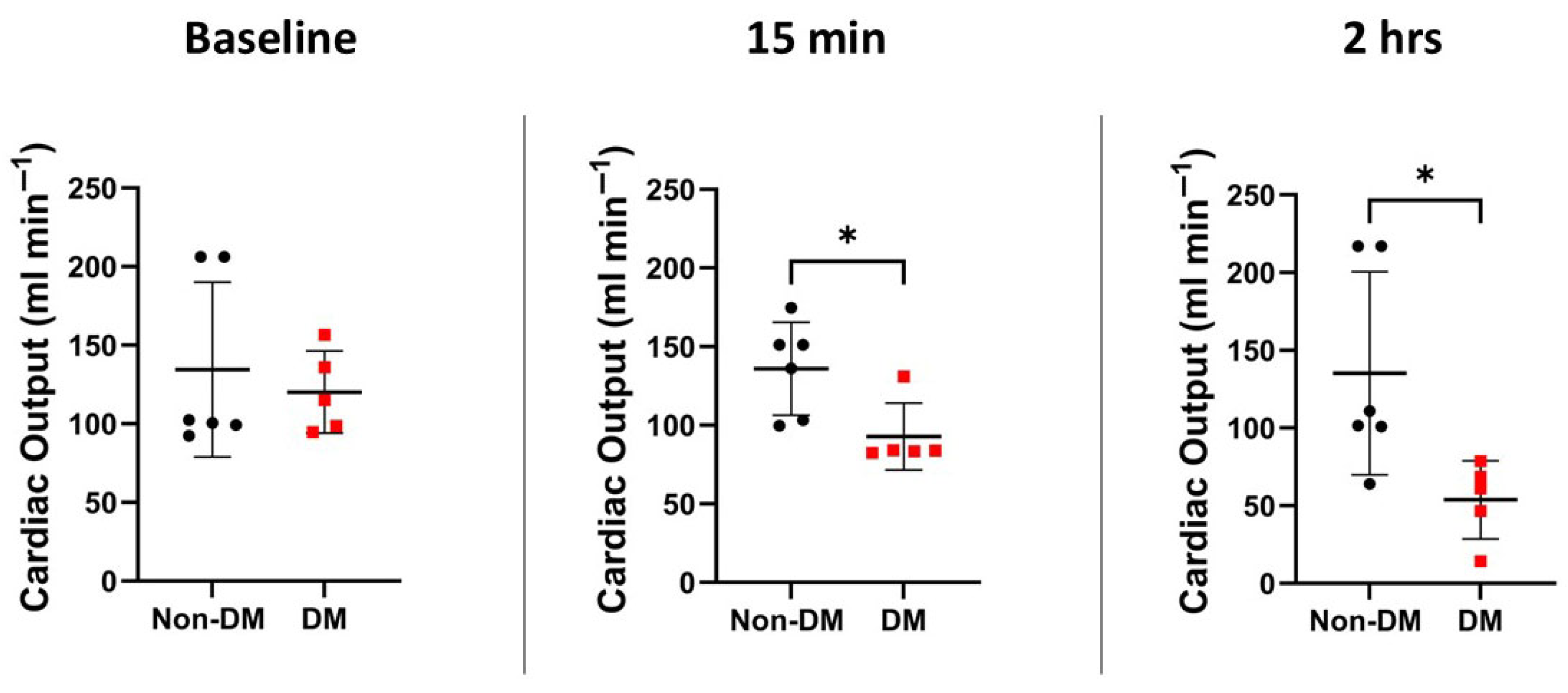
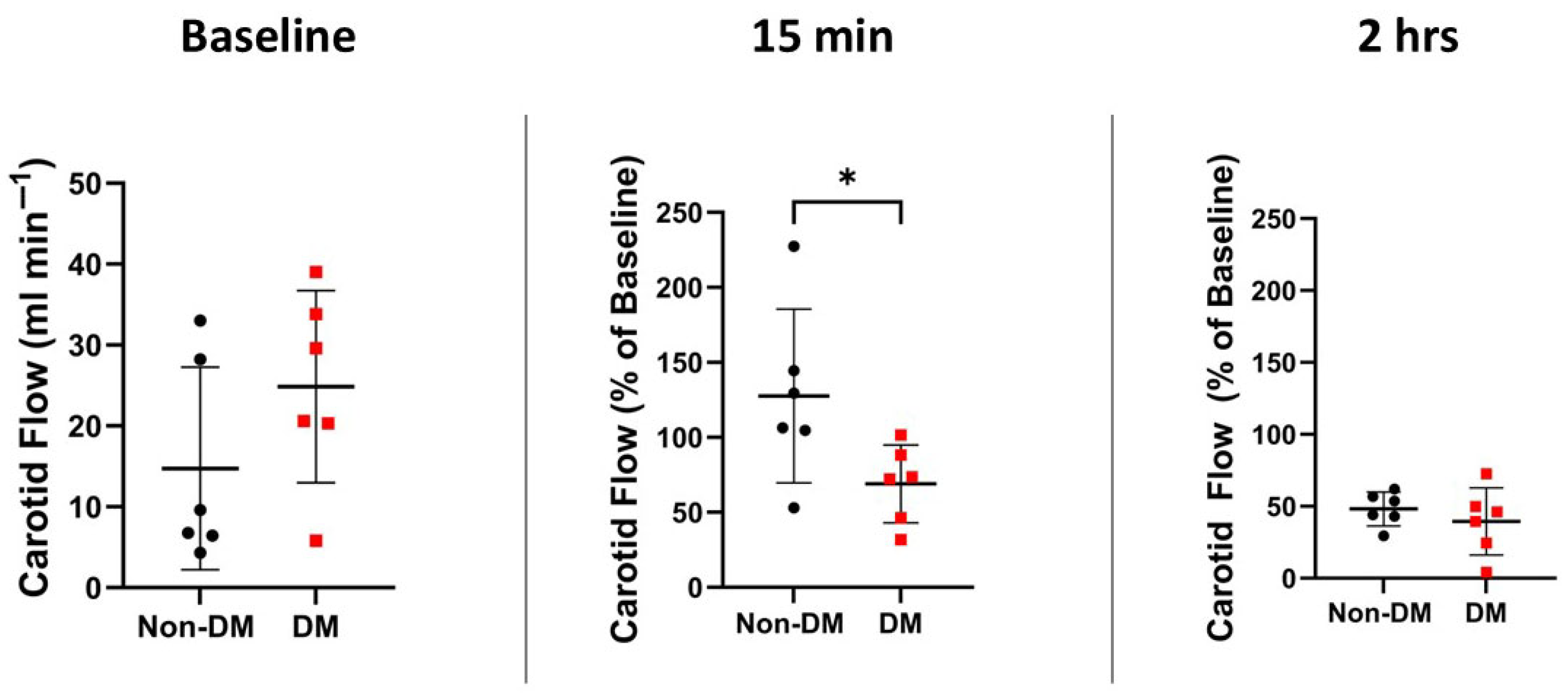
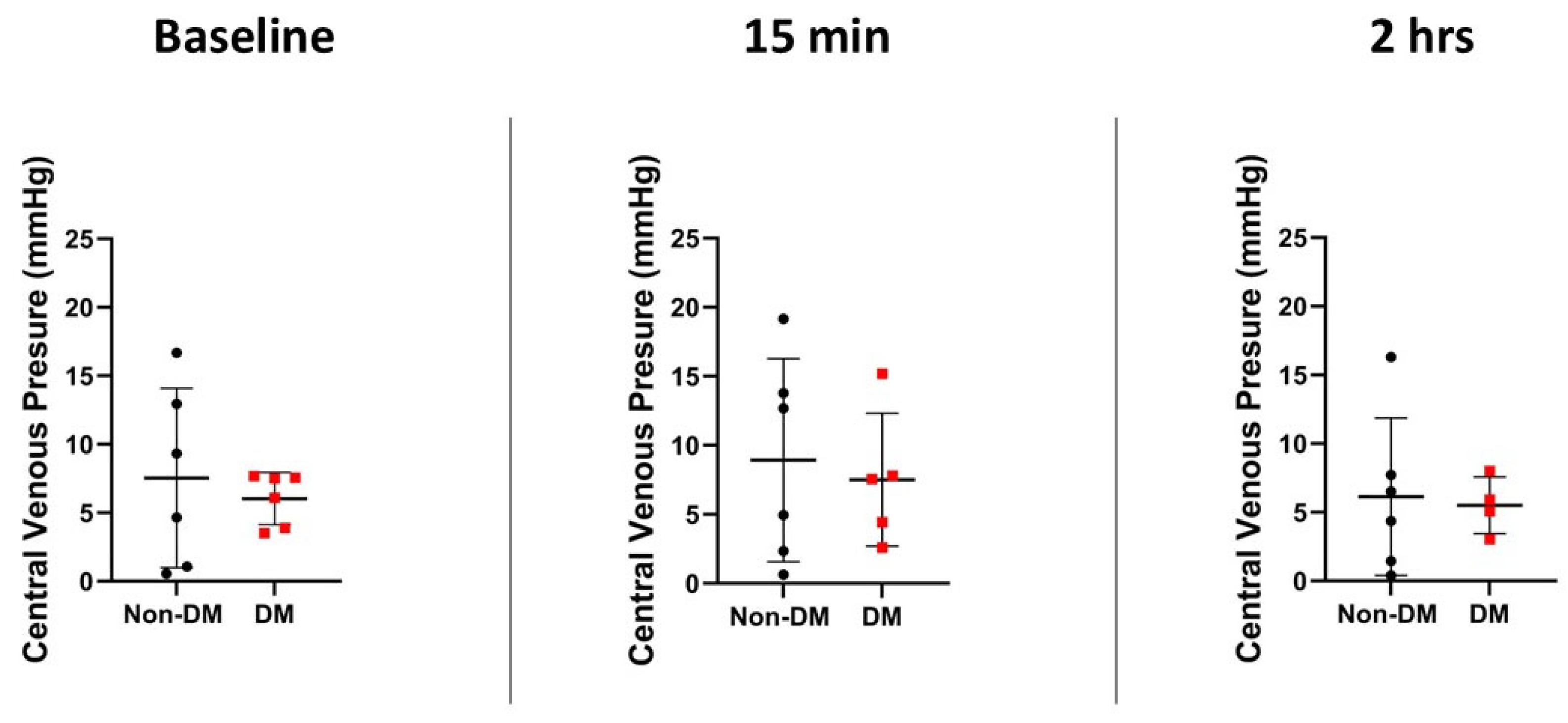
3.3. Hemodynamic Variables
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CPR | cardiopulmonary resuscitation |
| CVP | central venous pressure |
| DM | diabetes mellitus |
| h | hours |
| IQR | interquartile range |
| IRI | ischemia–reperfusion injury |
| MAP | mean arterial pressure |
| Mcg | microgram |
| Min | minutes |
| OHCA | out of hospital cardiac arrest |
| ROSC | return of spontaneous circulation |
| S | seconds |
| SGLT2 | Sodium–glucose cotransporter-2 |
| ZDF | Zucker diabetic fatty |
References
- Wong, M.K.Y.; Morrison, L.J.; Qiu, F.; Austin, P.C.; Cheskes, S.; Dorian, P.; Scales, D.C.; Tu, J.V.; Verbeek, P.R.; Wijeysundera, H.C.; et al. Trends in Short- and Long-Term Survival Among Out-of-Hospital Cardiac Arrest Patients Alive at Hospital Arrival. Circulation 2014, 130, 1883–1890. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.W.; Ersbøll, A.K.; Folke, F.; Wolthers, S.A.; Andersen, M.P.; Blomberg, S.N.; Andersen, L.B.; Lippert, F.; Torp-Pedersen, C.; Christensen, H.C. Training in Basic Life Support and Bystander-Performed Cardiopulmonary Resuscitation and Survival in Out-of-Hospital Cardiac Arrests in Denmark, 2005 to 2019. JAMA Netw. Open 2023, 6, e233338. [Google Scholar] [CrossRef] [PubMed]
- Majewski, D.; Ball, S.; Finn, J. Systematic review of the relationship between comorbidity and out-of-hospital cardiac arrest outcomes. BMJ Open 2019, 9, e031655. [Google Scholar] [CrossRef]
- Wiberg, S.; Holmberg, M.J.; Donnino, M.W.; Kjaergaard, J.; Hassager, C.; Witten, L.; Berg, K.M.; Moskowitz, A.; Andersen, L.W.; Grossestreuer, A.; et al. Age-dependent trends in survival after adult in-hospital cardiac arrest. Resuscitation 2020, 151, 189–196. [Google Scholar] [CrossRef]
- Rajan, S.; Folke, F.; Hansen, S.M.; Hansen, C.M.; Kragholm, K.; Gerds, T.A.; Lippert, F.K.; Karlsson, L.; Møller, S.; Køber, L.; et al. Incidence and survival outcome according to heart rhythm during resuscitation attempt in out-of-hospital cardiac arrest patients with presumed cardiac etiology. Resuscitation 2017, 114, 157–163. [Google Scholar] [CrossRef]
- Voruganti, D.C.; Chennamadhavuni, A.; Garje, R.; Shantha, G.P.S.; Schweizer, M.L.; Girotra, S.; Giudici, M. Association between diabetes mellitus and poor patient outcomes after out-of-hospital cardiac arrest: A systematic review and meta-analysis. Sci. Rep. 2018, 8, 17921. [Google Scholar] [CrossRef]
- Ro, Y.S.; Do Shin, S.; Song, K.J.; Lee, E.J.; Lee, Y.J.; Kim, J.Y.; Jang, D.B.; Kim, M.J.; Kong, S.Y. Interaction effects between hypothermia and diabetes mellitus on survival outcomes after out-of-hospital cardiac arrest. Resuscitation 2015, 90, 35–41. [Google Scholar] [CrossRef]
- Parry, M.; Danielson, K.; Brennenstuhl, S.; Drennan, I.R.; Morrison, L.J. The association between diabetes status and survival following an out-of-hospital cardiac arrest: A retrospective cohort study. Resuscitation 2017, 113, 21–26. [Google Scholar] [CrossRef]
- Patel, K.P. Volume reflex in diabetes. Cardiovasc. Res. 1997, 34, 81–90. [Google Scholar] [CrossRef]
- Lukovits, T.G.; Mazzone, T.; Gorelick, P.B. Diabetes mellitus and Cerebrovascular Disease. Neuroepidemiology 1998, 18, 1–14. [Google Scholar] [CrossRef]
- Gallwitz, B.; Kazda, C.; Kraus, P.; Nicolay, C.; Schernthaner, G. Contribution of insulin deficiency and insulin resistance to the development of type 2 diabetes: Nature of early stage diabetes. Acta Diabetol. 2013, 50, 39–45. [Google Scholar] [CrossRef]
- Li, W.A.; Moore-Langston, S.; Chakraborty, T.; Rafols, J.A.; Conti, A.C.; Ding, Y. Hyperglycemia in stroke and possible treatments. Neurol. Res. 2013, 35, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Johnston, K.C.; Hall, C.E.; Kissela, B.M.; Bleck, T.P.; Conaway, M.R. Glucose Regulation in Acute Stroke Patients (GRASP) Trial. Stroke 2009, 40, 3804–3809. [Google Scholar] [CrossRef] [PubMed]
- Bruno, A.; Kent, T.A.; Coull, B.M.; Shankar, R.R.; Saha, C.; Becker, K.J.; Kissela, B.M.; Williams, L.S. Treatment of Hyperglycemia In Ischemic Stroke (THIS). Stroke 2008, 39, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K.; Ramarao, P. Animal models in type 2 diabetes research: An overview. Indian J. Med. Res. 2007, 125, 451–472. [Google Scholar] [PubMed]
- Barajas, M.B.; Oyama, T.; Shiota, M.; Li, Z.; Zaum, M.; Zecevic, I.; Riess, M.L. Ischemic Post-Conditioning in a Rat Model of Asphyxial Cardiac Arrest. Cells 2024, 13, 1047. [Google Scholar] [CrossRef]
- Philipson, L.H. β-Agonists and metabolism. J. Allergy Clin. Immunol. 2002, 110 (Suppl. 6), S313–S317. [Google Scholar] [CrossRef]
- Zhang, M.; Lv, X.-Y.; Li, J.; Xu, Z.-G.; Chen, L. The characterization of high-fat diet and multiple low-dose streptozotocin induced type 2 diabetes rat model. Exp. Diabetes Res. 2008, 2008, 704045. [Google Scholar] [CrossRef]
- Guo, X.-x.; Wang, Y.; Wang, K.; Ji, B.-p.; Zhou, F. Stability of a type 2 diabetes rat model induced by high-fat diet feeding with low-dose streptozotocin injection. J. Zhejiang Univ. Sci. B 2018, 19, 559–569. [Google Scholar] [CrossRef]
- Reed, M.; Meszaros, K.; Entes, L.; Claypool, M.; Pinkett, J.; Gadbois, T.; Reaven, G. A new rat model of type 2 diabetes: The fat-fed, streptozotocin-treated rat. Metab. Clin. Exp. 2000, 49, 1390–1394. [Google Scholar] [CrossRef]
- Levy, B.; Clere-Jehl, R.; Legras, A.; Morichau-Beauchant, T.; Leone, M.; Frederique, G.; Quenot, J.-P.; Kimmoun, A.; Cariou, A.; Lassus, J. Epinephrine versus norepinephrine for cardiogenic shock after acute myocardial infarction. J. Am. Coll. Cardiol. 2018, 72, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Daviaud, F.; Dumas, F.; Demars, N.; Geri, G.; Bouglé, A.; Morichau-Beauchant, T.; Nguyen, Y.-L.; Bougouin, W.; Pène, F.; Charpentier, J.; et al. Blood glucose level and outcome after cardiac arrest: Insights from a large registry in the hypothermia era. Intensive Care Med. 2014, 40, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Maitland, A.; Weisel, R.D.; Li, S.-H.; Fedak, P.W.M.; Pomroy, N.C.; Mickle, D.A.G.; Li, R.-K.; Ko, L.; Rao, V. Hyperglycemia exaggerates ischemia-reperfusion–induced cardiomyocyte injury: Reversal with endothelin antagonism. J. Thorac. Cardiovasc. Surg. 2002, 123, 1120–1124. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.J.; Shiota, M.; Li, Z.; Barajas, M.B.; Oyama, T.; Riess, M.L. Abstract 176: The Pathological Role of Aldose Reductase in Isolated Cardiomyocytes Undergoing Hypoxia/Reoxygenation After Prior Exposure to High Glucose Concentrations. Circulation 2023, 148 (Suppl. 1). [Google Scholar] [CrossRef]
- Balzer, C.; Cleveland, W.J.; Li, Z.; Riess, M.L. Buffer glucose adjustment affects myocardial function after ischemia–reperfusion in long-term diabetic rat isolated hearts. Physiol. Rep. 2022, 10, e15387. [Google Scholar] [CrossRef]
- Penna, C.; Andreadou, I.; Aragno, M.; Beauloye, C.; Bertrand, L.; Lazou, A.; Falcão-Pires, I.; Bell, R.; Zuurbier, C.J.; Pagliaro, P.; et al. Effect of hyperglycaemia and diabetes on acute myocardial ischaemia–reperfusion injury and cardioprotection by ischaemic conditioning protocols. Br. J. Pharmacol. 2020, 177, 5312–5335. [Google Scholar] [CrossRef]
- Selker, H.P.; Beshansky, J.R.; Sheehan, P.R.; Massaro, J.M.; Griffith, J.L.; D’Agostino, R.B.; Ruthazer, R.; Atkins, J.M.; Sayah, A.J.; Levy, M.K.; et al. Out-of-Hospital Administration of Intravenous Glucose-Insulin-Potassium in Patients With Suspected Acute Coronary Syndromes: The IMMEDIATE Randomized Controlled Trial. JAMA 2012, 307, 1925–1933. [Google Scholar] [CrossRef]
- Okoduwa, S.I.R.; Umar, I.A.; James, D.B.; Inuwa, H.M. Appropriate insulin level in selecting fortified diet-fed, streptozotocin-treated rat model of type 2 diabetes for anti-diabetic studies. PLoS ONE 2017, 12, e0170971. [Google Scholar] [CrossRef]
- Del Prato, S.; Marchetti, P.; Bonadonna, R.C. Phasic Insulin Release and Metabolic Regulation in Type 2 Diabetes. Diabetes 2002, 51 (Suppl. 1), S109–S116. [Google Scholar] [CrossRef]
- Hoxworth, J.M.; Xu, K.; Zhou, Y.; Lust, W.D.; LaManna, J.C. Cerebral metabolic profile, selective neuron loss, and survival of acute and chronic hyperglycemic rats following cardiac arrest and resuscitation. Brain Res. 1999, 821, 467–479. [Google Scholar] [CrossRef]
- Vammen, L.; Rahbek, S.; Secher, N.; Povlsen, J.A.; Jessen, N.; Løfgren, B.; Granfeldt, A. Type 2 diabetes mellitus worsens neurological injury following cardiac arrest: An animal experimental study. Intensive Care Med. Exp. 2018, 6, 23. [Google Scholar] [CrossRef]
- Balzer, C.; Baudenbacher, F.; Salzman, M.M.; Cleveland, W.J.; Eagle, S.; Riess, M.L. Abstract 328: Hemodynamic Comparison of Zucker Diabetic Fatty Rats to Their Lean Littermates After Asphyxial Cardiac Arrest. Circulation 2018, 138 (Suppl. 2). [Google Scholar] [CrossRef]
- Miao, Y.; Zhang, W.; Zhong, Y.; Zhong, M.; Ma, X. Diastolic function of the right ventricle is impaired in experimental type 2 diabetic rat models. Turk. J. Med. Sci. 2014, 44, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Zhao, Y.; Wang, L.; Peng, X.; Li, H.; Zhao, Y.; He, Y.; Shao, H.; Zhong, X.; Li, H.; et al. H2S restores the cardioprotection from ischemic post-conditioning in isolated aged rat hearts. Cell Biol. Int. 2015, 39, 1173–1176. [Google Scholar] [CrossRef] [PubMed]
- Daniels, A.; Linz, D.; van Bilsen, M.; Rütten, H.; Sadowski, T.; Ruf, S.; Juretschke, H.-P.; Neumann-Haefelin, C.; Munts, C.; van der Vusse, G.J.; et al. Long-term severe diabetes only leads to mild cardiac diastolic dysfunction in Zucker diabetic fatty rats. Eur. J. Heart Fail. 2012, 14, 193–201. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, C.; Yu, S.; Luo, Z.; Chen, Y.; Liu, Q.; Hua, F.; Xu, G.; Yu, P. Sevoflurane Postconditioning Protects Rat Hearts against Ischemia-Reperfusion Injury via the Activation of PI3K/AKT/mTOR Signaling. Sci. Rep. 2014, 4, 7317. [Google Scholar] [CrossRef]
- Wang, M.; Hua, T.; Zhang, Y.; Huang, Q.; Shi, W.; Chu, Y.; Hu, Y.; Pan, S.; Ling, B.; Tang, W.; et al. Effects of canagliflozin preconditioning on post-resuscitation myocardial function in a diabetic rat model of cardiac arrest and cardiopulmonary resuscitation. Eur. J. Pharmacol. 2025, 988, 177212. [Google Scholar] [CrossRef]
- Chen, N.; Callaway, C.W.; Guyette, F.X.; Rittenberger, J.C.; Doshi, A.A.; Dezfulian, C.; Elmer, J. Arrest etiology among patients resuscitated from cardiac arrest. Resuscitation 2018, 130, 33–40. [Google Scholar] [CrossRef]
- Uray, T.; Dezfulian, C.; Palmer, A.A.; Miner, K.M.; Leak, R.K.; Stezoski, J.P.; Janesko-Feldman, K.; Kochanek, P.M.; Drabek, T. Cardiac Arrest Induced by Asphyxia Versus Ventricular Fibrillation Elicits Comparable Early Changes in Cytokine Levels in the Rat Brain, Heart, and Serum. J. Am. Heart Assoc. 2021, 10, e018657. [Google Scholar] [CrossRef]
- Guo, J. Comparison between two rat models of cardiac arrest: Asphyxiation and ventricular fibrillation. J. Shanghai Jiaotong Univ. (Med. Sci.) 2018, 12, 380–385. [Google Scholar]
- Padhi, S.; Nayak, A.K.; Behera, A. Type II diabetes mellitus: A review on recent drug based therapeutics. Biomed. Pharmacother. 2020, 131, 110708. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Gu, Y.; Hu, Y.; Ji, Z.; Wang, S.; Lin, Z.; Li, X.; Xie, Z.; Pan, S. Glibenclamide improves survival and neurologic outcome after cardiac arrest in rats. Crit. Care Med. 2015, 43, e341–e349. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, T.E.; Jia, L.; Blom, M.T.; Verkerk, A.O.; Devalla, H.D.; Boink, G.J.J.; Souverein, P.C.; de Boer, A.; Tan, H.L. Sulfonylurea antidiabetics are associated with lower risk of out-of-hospital cardiac arrest: Real-world data from a population-based study. Br. J. Clin. Pharmacol. 2021, 87, 3588–3598. [Google Scholar] [CrossRef] [PubMed]
- Júlíusdóttir, Y.K.; Halili, A.; Coronel, R.; Folke, F.; Torp-Pedersen, C.; Gislason, G.H.; Eroglu, T.E. Sodium-glucose cotransporter-2 inhibitors compared with glucagon-like-peptide-1 receptor agonists and out-of-hospital cardiac arrest in type 2 diabetes: A nationwide nested case-control study. Eur. Heart J. Cardiovasc. Pharmacother. 2023, 9, 437–443. [Google Scholar] [CrossRef]
- Paolisso, P.; Bergamaschi, L.; Gragnano, F.; Gallinoro, E.; Cesaro, A.; Sardu, C.; Mileva, N.; Foà, A.; Armillotta, M.; Sansonetti, A.; et al. Outcomes in diabetic patients treated with SGLT2-Inhibitors with acute myocardial infarction undergoing PCI: The SGLT2-I AMI PROTECT Registry. Pharmacol. Res. 2023, 187, 106597. [Google Scholar] [CrossRef]
- Nikolic, M.; Zivkovic, V.; Jovic, J.J.; Sretenovic, J.; Davidovic, G.; Simovic, S.; Djokovic, D.; Muric, N.; Bolevich, S.; Jakovljevic, V. SGLT2 inhibitors: A focus on cardiac benefits and potential mechanisms. Heart Fail. Rev. 2022, 27, 935–949. [Google Scholar] [CrossRef]
- Strain, W.D.; Paldánius, P.M. Diabetes, cardiovascular disease and the microcirculation. Cardiovasc. Diabetol. 2018, 17, 57. [Google Scholar] [CrossRef]
- Oltman, C.L. Complications in the Coronary Circulation Associated with Diabetes. In Studies in Diabetes; Obrosova, I., Stevens, M.J., Yorek, M.A., Eds.; Springer: New York, NY, USA, 2014; pp. 37–47. [Google Scholar]
- From, A.M.; Scott, C.G.; Chen, H.H. Changes in Diastolic Dysfunction in Diabetes Mellitus Over Time. Am. J. Cardiol. 2009, 103, 1463–1466. [Google Scholar] [CrossRef]
- Galderisi, M. Diastolic Dysfunction and Diabetic Cardiomyopathy. J. Am. Coll. Cardiol. 2006, 48, 1548–1551. [Google Scholar] [CrossRef]
- Ahmadieh, H.; Azar, S.T. Liver disease and diabetes: Association, pathophysiology, and management. Diabetes Res. Clin. Pract. 2014, 104, 53–62. [Google Scholar] [CrossRef]
- Colhoun, H.M.; Lee, E.T.; Bennett, P.H.; Lu, M.; Keen, H.; Wang, S.L.; Stevens, L.K.; Fuller, J.H.; the WHOMSG. Risk factors for renal failure: The WHO multinational study of vascular disease in diabetes. Diabetologia 2001, 44, S46. [Google Scholar] [CrossRef][Green Version]
- Phipps, M.S.; Jastreboff, A.M.; Furie, K.; Kernan, W.N. The Diagnosis and Management of Cerebrovascular Disease in Diabetes. Curr. Diabetes Rep. 2012, 12, 314–323. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barajas, M.B.; Oyama, T.; Shiota, M.; Li, Z.; Zaum, M.; Zecevic, I.; Riess, M.L. Diabetes Worsens Outcomes After Asphyxial Cardiac Arrest in Rats. Diabetology 2025, 6, 78. https://doi.org/10.3390/diabetology6080078
Barajas MB, Oyama T, Shiota M, Li Z, Zaum M, Zecevic I, Riess ML. Diabetes Worsens Outcomes After Asphyxial Cardiac Arrest in Rats. Diabetology. 2025; 6(8):78. https://doi.org/10.3390/diabetology6080078
Chicago/Turabian StyleBarajas, Matthew B., Takuro Oyama, Masakazu Shiota, Zhu Li, Maximillian Zaum, Ilija Zecevic, and Matthias L. Riess. 2025. "Diabetes Worsens Outcomes After Asphyxial Cardiac Arrest in Rats" Diabetology 6, no. 8: 78. https://doi.org/10.3390/diabetology6080078
APA StyleBarajas, M. B., Oyama, T., Shiota, M., Li, Z., Zaum, M., Zecevic, I., & Riess, M. L. (2025). Diabetes Worsens Outcomes After Asphyxial Cardiac Arrest in Rats. Diabetology, 6(8), 78. https://doi.org/10.3390/diabetology6080078







