Abstract
According to the National Institutes of Health, approximately 465 million individuals are affected by type II diabetes mellitus (T2DM) and could benefit from managing their condition with a high-quality diet based on proper, nutrient-rich food choices. A plant-based diet not only has health benefits but also helps mitigate climate change by reducing greenhouse gas emissions, but the Mediterranean diet has the most beneficial effect on overall health. In contrast, ultra-processed foods have a negative impact on T2DM outcomes. Reviewing the nutritional profile of different meals, snacks and desserts would be helpful in enhancing their quality, strengthening the role of dietitians and doctors and protecting against T2DM complications. This approach would also increase simplification and education for consumers. The PubMed-Medline, Web of Science, Scopus and Cochrane Library databases were searched for relevant articles published up to May, from 2000 (based on publication date). The results support the need to reinforce health claims and highlight public demand for food choices while also improving patient quality of life.
1. Introduction
Type II diabetes mellitus (T2DM) develops due to a combination of insulin resistance and inadequate insulin secretion [1]. The pathogenesis is only partly understood, but it is heterogeneous, and both genetic factors that influence insulin release and sensitivity, as well as lifestyle and environmental factors such as obesity play an important role [2].
A total of 13.2% of all US adults had diabetes during 2017–2020 [2], and by 2050, more than 1 billion people are projected to have diabetes [3]. As for the parameters that play a crucial role in T2DM during childhood and adolescence, four important studies were conducted: GENESIS [4], ToyBox [5], Feel4Diabetes [6] and EMENO [7] studied the prevalence of T2DM and type I diabetes mellitus (T1DM) in adults in Greece. Irrespective of age, origin or educational background [8], a patient with T2DΜ is at an increased risk of complications due to metabolic disorders and interrelated risk factors [9,10,11,12,13,14,15,16,17]. The pathogenesis, including genetic susceptibility, environmental and occupational factors, tobacco use, high alcohol consumption, high body mass index (BMI), dietary factors and low physical activity, has been extensively studied [3].
Although educating diabetic patients is an essential part of diabetes management [1], self-monitoring is also of great benefit. In addition, dietary patterns have been analyzed worldwide to identify dietary strategies that could lead to improvement in postprandial glucose levels, glucose stability, and lipid biomarkers in patients with T2DM [18]. Undoubtedly, nutrition serves as an umbrella term [19], and foods should be analyzed not only for their nutritional profiles but also for the mechanisms by which their bioactive compounds achieve glucose stability, which is a comprehensive approach to T2DM nutritional management [20]. Furthermore, the use of medication could help achieve optimal glycemic control [21,22].
Culinary medicine is a lifestyle approach that links the functionality of foods [20] with the science of nutrition to promote health and prevent or manage diet-related diseases [23]. This approach may improve quality of life by helping patients make easier food choices [1] based on their preferences. However, meal testing to provide more diabetic-friendly food choices for patients is not a new concept due to the fact that medical nutrition therapy (MNT) has existed since 2022 [24].
Although many diet patterns have been proposed, a clear research question is if there is one that links nutrition, dietetics and functional foods for the treatment of the disease and prevention of its complications. The answer would have particular value because it could be a facilitator of patients’ choices while also improving their quality of life. This study will discuss the clinical profile, with a particular focus on glycemic markers, following the consumption of meals or snacks in adults with diabetes mellitus. It will evaluate their impact on T2DM outcomes based on nutritional profile, timing of consumption and preparation methods. To our knowledge, no other review has examined these parameters—such as nutritional profile, timing and preparation—alongside the effects of dietary interventions and meal alternatives on the biochemical markers of patients with T2DM. The findings of this study may provide an opportunity to reassess newer guidelines and other regulations concerning diabetic food products, strengthen health claims in various countries and address public demand for foods with health claims.
2. Methods
2.1. Review Aim and Strategy
This review was conducted following the Preferred Reporting items for Reviews (PRISMA) 2020 guidelines and checklist (Figure 1) [25]. This review aimed to explore if there is one that links nutrition, dietetics and functional foods for the treatment of the disease and prevention of its complications. The answer would have particular value because it could be a facilitator of patients’ choices while also improving their quality of life.
2.2. Literature Search, Study Selection, Eligibility Criteria and Quality Assessment
The search strategy involved a comprehensive examination of the following databases:
PubMed and Web of Science. Searches utilized MeSH terms and keywords, specifically targeting “#Type 2 diabetes mellitus,” “#Diet,” “#Interventions”, “#Snacks” “Meals,” “#Nutritional profile,” “#Nutrients,” “#Randomized clinical trial” and “#Bioactive compounds.” The aim was to investigate the relationship between the nutritional profile of different diet patterns, meals and snacks, with a particular focus on micronutrients, macronutrients and their impact on health.
Additional searches were conducted in PubMed, Web of Science, Scopus and the Cochrane Library using similar keywords, including “#Snacks,” “#Meals,” “#Bioactive compounds,” “#Health benefits,” “#Type 2 diabetes mellitus,” “#Hyperglycemia,” “#Obesity,” “#Nutrition,” “#Diet” and “#Randomized clinical trial.” Other terms such as “#Food” and “#Nutrition” were also included to ensure a broader scope. The objective was to explore dietary habits, nutritional interventions and the role of various bean types in modulating specific bioactive compounds and addressing related disorders.
The Scopus and Cochrane Library searches emphasized “#Diet,” “#Interventions,” “#Nutrition,” “#Type 2 diabetes mellitus,” “#Side effects” and “#Randomized clinical trial,” with the additional keyword “#Food.” The primary aim was to identify articles examining the association between food consumption and adverse symptoms, with particular attention given to the guidance provided by health professionals, since the rate of the problem has been confirmed [26]. The selection of these databases was based on their reputability, comprehensiveness and relevance to healthcare and medical research (Table 1). The inclusion and exclusion criteria are summarized in Table 2.

Table 1.
Details regarding the search process and unique contributions of each database to this study.

Table 2.
Inclusion and exclusion criteria.
2.3. Outcome Measures
The primary outcome measure was the mean change in glycated hemoglobin (HbA1c) and fasting glucose between the intervention and comparator groups. Secondary outcome measures included the following:
1. Biochemical outcomes: Changes in lipid profile, post-prandial glucose, hormones etc.
2. Body anthropometry outcomes: Changes in body weight, body mass index (BMI) and body fat percentage.
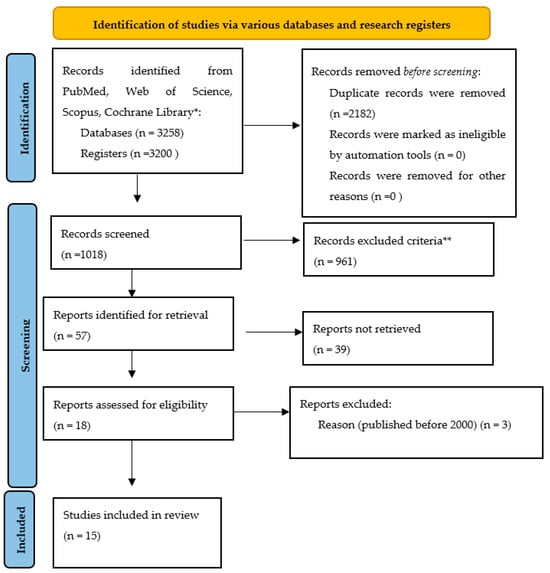
Figure 1.
Prisma flow chart of this study (version 2020) [27,28,29,30,31,32,33,34,35,36,37,38,39,40,41]. * PubMed 1203, Web of Science 4, Scopus 2000, Cochrane Library 51 ** Table 2.
3. Results
3.1. Dietary Interventions
This study examines the diet and eating habits of the patients. In this respect, the study differs from others that follow a similar pattern (Table 3) by emphasizing the role of food in function and examining the extent to which changes in human eating habits [19] can promote better nutrition, which is important for biomarkers relevant to T2DM. The Mediterranean diet is considered a healthy diet, and the extent to which changes in human eating habits at the end of the 20th century could be beneficial for T2DM patients has been investigated [26]. The prevalence of diabetes in Greece according the results of the First National Survey of Morbidity and Risk Factors (EMENO) study was 11.9% (95% CI: 10.9–12.9), known diabetes 10.4% (9.5–11.4) and undiagnosed diabetes 1.5% (1.1–1.9) [7].

Table 3.
Dietary interventions for T2DM are crucial for managing the disease.
The energy intake ranged from 1500 kcal per day to 1800 kcal per day [29]. The snack energy intake could range from 200–300 kcal [30], and ultra-processed food consumption was minimized [42]. Omega-3 fatty acids ranged from 56 mg/day to 98 mg/day [34,43]. No differences in the response to dietary interventions were observed across age, BMI and gender, but the duration from the T2DM diagnosis seemed to be a factor that could play a major role. Moreover, sustainability is a term that could and should be mentioned for the plant-based diets but also for interventions or diet patterns that are based on local and seasonal products.
3.2. Nutritional Profile
3.2.1. Snacks (Snack Bars, Homemade Cookies, Etc.)
Vegetables and fruits are an important part of a healthy eating pattern [29]. All snack bars share a common feature in their nutritional profile: a high amount of dietary fibers [44,45,46,47,48,49,50,51,52,53,54,55,56,57]. Although the consumption of snacks between the main meals is important in patient care [58], only a few clinical trials have investigated the acute metabolic effects of snacks in T2DM patients [44,45,51,52,59,60,61]. Although it would be beneficial to be rich in protein and low in saturated fats [62], the differences among meal choices lie not only in the percentage of protein content, which ranged from 2.5 g/100 g [45,50] to 21.95 g/100 g [51,63], but also in the carbohydrate content (1.3 g/100 g [52] −45.1 g/100 g) [46], and there is concern regarding the fact that snack bars can have 16.81 g/100 g [64] and muffins 17.3 g/100 g [49], which is a big snack. Side effects from snack bars such as bloating, nausea, etc., have been mentioned by participants [65]. Kouvari et al. highlights the need to avoid ultra-processed snacks [42]. Barnard et al. [39] calculated the nutrient contents such as vitamins and minerals from fruit and vegetable intakes for individuals completing the intervention, and vitamins K, C and B-6 were higher for the vegan group after the intervention, but adverse effects were observed for vitamin B-12, calcium and zinc.
3.2.2. Meals (Pies, Formulas, Etc.)
Almost all studies followed the recommendations of the American Diabetes Association (ADA) for the main meals, which usually included lean meat such as seafood and chicken or legumes with vegetables and whole grains in different portions in order to achieve the percentage of each pattern’s nutrient intake [27,28,29,30,31,32,33,34,35,36,37,38,40]. Kavanagh et al. proposed a diet pattern with <7% saturated fat and <200 mg of dietary cholesterol a day. Low-glycemic index foods are those that cause a slower, more gradual rise in blood sugar levels when eaten and are recommended [36]. Carbohydrates exhibit the highest concentration in liquid meal replacements [66], while dietary fiber is also present in significant amounts. The protein content ranges from 21 to 25 g per meal, whereas the fat content varies from 14.7 g [67] to 15 g. Greek vegetarian plates are notable for their low fat content [67].
3.3. Timing
Breakfast is often described as the most important meal of the day [68], and skipping this morning meal is really detrimental to health according to a recent study. Moreover, the results showed that participants on the later meal schedule woke up feeling hungrier, exhibited lower calorie expenditure and had adipose tissue changes indicative of increased fat storage. A previous study also found that eating four hours later significantly impacted hunger levels, postprandial energy metabolism and fat storage. Moreover, late-night eating has been linked to sleep disturbances and blood sugar spikes, making it advisable to avoid whenever possible [69].
3.4. Preparation
Plant-based chemicals that are not derived from animal sources and are known as phytochemicals act as antioxidants and anti-inflammatories, helping to reduce inflammation and counteract its harmful effects. In some cases, such as green leafy vegetables [70] and other vegetables [71], higher cooking temperatures can release more phytochemicals and enhance their antidiabetic properties [72,73]. Dried fruits aid digestion by providing fiber, which promotes intestinal health and prevents constipation. They are also rich in fiber, which aids digestion and promotes regular bowel movements [74]. Although the drying process slightly reduces some heat-sensitive micronutrients such as vitamin C, the majority of essential vitamins, nutrients and minerals are preserved [75]. For this reason, researchers have incorporated antioxidant-rich fruits and berries—such as raisins, dates [46] and dried cranberries—into snack bars and other functional snacks [54]. As a result, these foods offer additional benefits due to their anti-inflammatory plant phenols [50]. Similarly, leafy green vegetables have been added to anti-diabetic blended meals to increase their health benefits. As dried fruit is higher in calories and sugar than its fresh counterparts, portion sizes should be moderated [73]. In some cases, refrigerating or freezing toast or bread has a similar effect to heat treatment by reducing moisture and thus making the bread last longer, although the methods are different [73,76]. However, this process also lowers the glycemic index of the food, making it more suitable for T2DM patients [52,76,77] while contributing to lower body weight [77]. Nevertheless, this does not mean that bread should be completely eliminated from the diet [46,76,77]. It should be noted that processed products usually contain more sugar and calories [78]. Therefore, cooking at home is recommended to maintain nutritional quality and minimize nutrient losses [79]. In addition, preparing meals at home allows for better portion control, which helps to maintain an ideal body mass index (BMI) [78].
3.5. Missing Points
This review found that most studies focused on the use of low-glycemic ingredients such as natural sweeteners, fruits and nuts in the formulation of snacks but did not include an intervention with the daily consumption of these snacks to assess biomarkers relevant to T2DM management [45,46,47]. Exceptions are the studies by Mustad et al. (7-day–14-day intervention) [48] and Christiansen et al. (eight-week intervention periods) [50]. In addition, Nurdin et al. was the only study to investigate both the glycemic index of a snack bar in healthy adults [53] and its effects on biomarkers in patients with T2DM [44]. On the other hand, the potential of alternative protein sources, such as mushroom powder [65] or nuts [55,65], has not been extensively investigated [80]. According to the EFSA, it is crucial for T2DM patients to reduce total fat intake to <30% of daily energy expenditure, limit saturated fats (including trans fats) to <10%, increase fiber intake to at least 40 g/day [23] and increase ω-3 fatty acid intake [20]. Achieving these dietary goals consistently with conventional foods can be challenging over time [1]. The EFSA considers a daily intake of more than 25 g of dietary fiber to be sufficient to reduce diabetes-related complications such as coronary heart disease [81] and to improve weight maintenance, which is associated with better postprandial glucose regulation [82]. With regard to the total fat content, the results are inconclusive and further research is needed, particularly in relation to legal guidelines for fat utilization [23]. The authors should critically re-evaluate the results of the diet interventions and consider the extent to which these results are consistent with their working hypotheses and whether they could be of benefit to a wider population (Table 4) with less restrictive inclusion criteria as part of a daily dietary plan. Future research directions should also investigate the applicability of these results to different types of diabetes mellitus.

Table 4.
The changes in biomarkers found in studies included in this research that are associated with increased T2DM consequences.
Diabetes is a cardiometabolic disorder in which both microvascular and macrovascular complications contribute to morbidity and mortality [83]. Figure 2 shows how nutritional interventions can minimize these complications with certain impacts on biomarkers.
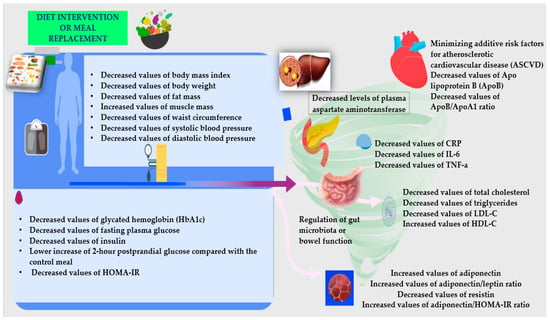
Figure 2.
The dietary interventions’ impact on biomarkers for patients with T2DM [27,28,29,30,31,32,33,34,35,36,37,38,39,40,41].
4. Discussion
The rapid evolution of human societies has led to an increased incidence of metabolic disorders. This recent scientific research (from 2000 to 2025) on nutritional therapy for adults with T2DM [83] links nutrition, dietetics and functional foods for the treatment of the disease and prevention of its complications [18]. It has particular value because it could be a facilitator of patients’ choices while also improving their quality of life. Medical nutrition therapy (MNT) is a key component of diabetes education and management [84].
When it comes to weight control, there is no one size fits all. There is no ideal macronutrient ratio for weight loss, but hypocaloric diets with different macronutrient compositions can be effective as long as they are consistent with dietary recommendations. Replacing one or two meals per day with formulated meal replacements is acceptable. Individuals who are overweight or obese may even achieve remission of T2DM without having to take antidiabetic drugs, provided they have lost sufficient weight [85]—approximately 0.5–9.8 kg (Table 4) [29,30,31,32,33,34,35,36,37,38,39,40,41]. The clinical evidence from this study supports this: the Diabetes Excess Weight Loss study showed no reversal of diabetes in 2 years [38] with a ketogenic diet, while the control group lost weight (2–3 kg, p < 0.001) and reduced their waist circumference (2–3 cm, p < 0.001). In the DIRECT study [29], a low-carbohydrate diet was found to be more effective than a low-fat diet for weight loss and improving HDL and triglycerides. Intermittent fasting studies [37] show an improvement in HbA1c levels even without weight loss. A Mediterranean diet with higher fat and lower carbohydrate intakes leads to lower mortality [30], and biomarkers such as HbA1c and LDL cholesterol are minimized and HDL cholesterol is increased [27,28,29,30,39]. HbA1c, two-hour second glucose and serum alanine aminotransferase (ALT) were lower in most studies with low carbohydrate intake [29,33], and it would be helpful to count baseline carbohydrates [86]. The studies in this review were based on guidelines such as whole grains, low-fat dairy products, high-fructose fruit smoothies and six small meals per day. Meanwhile, people are reversing their insulin resistance and improving their lipid levels with low-carb, ketogenic and carnivorous diets; time-restricted eating or intermittent fasting; strategic supplementation; and lifestyle changes before taking medications. On the other hand, many professional organizations—the ADA [87] and the American Heart Association (AHA) [88]—still adhere to old, industry-supported advice, which may be because eating packaged cereals, seed oils and snacks is not only convenient but also more acceptable to patients with T2DM than promoting protein, unsaturated fatty acids and metabolic flexibility. According to the results of this review, patients should shed their fear of saturated fat and become more sensitive to carbohydrate consumption to avoid the consequences of chronic hyperinsulinemia and metabolic dysfunction. Clinicians, dietitians and caregivers have already found that patients who try non-processed foods, strategic restriction and nutrient support have better glycemic biomarker outcomes than patients who stick to the standard script.
Individual foods are part of a diet characterized by their nutrient content, food groups and times of consumption [19]. According to the studies included in this review (Figure 1 and Table 1 and Table 2), most of them introduce an individualized dietary approach that emphasizes carbohydrate restriction over fat restriction, the substitution of SFAs with MUFAs and PUFAs and adequate intake of dietary fiber, all of which are key factors for optimizing diabetes management (Table 3) [89]. Previous interventions have suggested the adoption of the DASH diet not just for diabetic patients as a behavioral intervention for weight loss [90] nor simply as an ideal dietary pattern for T2DM patients, but rather, for hypertension management [91]. Vegan and Mediterranean dietary patterns may be effective in improving glycemic control in T2DM patients, and intermittent fasting, although promising, requires further investigation [92]. Another important finding highlighted in this review is the concern for nutrient intake [93]. Thus, it is not only the dietary pattern that matters but also the amount of consumption of each food [94], as well as other factors [95,96,97] that should be considered to achieve a healthy body weight and a high-quality diet [94,98] from childhood to prevent T2DM later in life. At a younger age, it can be difficult to prevent unhealthy eating habits as children often follow their parents’ dietary patterns [99,100]. Our findings demonstrate that lifestyle modifications, including physical activity combined with diet and nutrition, can lead to significant anthropometric changes that result in improvements in abdominal adiposity, as measured by waist circumference (WC), and in certain inflammatory markers in overweight/obese individuals with T2DM [92] and other biomarkers. However, further high-quality research is needed to clarify the underlying mechanisms by which these interventions affect inflammatory markers in this population [101].
Not only the nutrient profile of diets but also the ingredients of many foods are responsible for the acute effects on postprandial glucose and insulin levels. Many of these ingredients, such as mushrooms, fruits (e.g., cranberries, grapes) and vegetables, have been labeled “functional foods,” and their mechanisms of action have been extensively studied [72].
Functional foods are divided into the following categories based on their effects: (1) those that contain an increased concentration of a beneficial ingredient, such as phytosterols or dietary fiber; (2) those that contain an additional beneficial ingredient, such as vitamins, minerals or probiotic cultures; (3) those that help eliminate negative ingredients, such as allergens; and (4) those in which a negative ingredient has been partially replaced by a positive one, such as fat substitutes [102]. Several brands have attempted to improve their health claims by, for example, offering a portion of daily fiber or fruit intake per bar or containing less than 100 calories [65]. However, few have conducted clinical trials in patients with type 2 diabetes mellitus [103]. Studies suggest that 40–48% of patients aged 40–70 years consume foods designed for diabetics to reduce the complications associated with the disease (Figure 2) [72].
In the early 1980s, the UK was one of the first countries to establish new criteria for “diabetic foods,” clarifying that for a product to be labeled as “suitable for diabetics,” it must not contain more fat or energy than its conventional counterpart. Today, according to the EFSA, a food is considered suitable for diabetics if it has a low glycemic load (compared to 50 g of anhydrous glucose) and if its daily consumption over a period of at least three months leads to a positive change in the HbA1c value. Special foods for diabetics are often more expensive than conventional low-sugar products. The sweeteners used in these products are generally considered safe if they are consumed within the recommended daily intake. These include both natural sugar substitutes (xylitol, mannitol, isomalt and maltitol) and artificial sweeteners (saccharin, aspartame, sucralose, acesulfame K and cyclamate), which are regulated by the EFSA [104]. The following are dietary recommendations for diabetes management:
- -
- Diabetes and Nutrition Study Group of the European Association for the Study of Diabetes (EASD). European recommendations for the dietary management of diabetes (2023): “Intake of free or added sugars should be less than 10% of total energy intake. Non-nutritive sweeteners can be used to replace sugar in foods and beverages” [87,105].
- -
- Medical Nutrition Therapy Recommendations (2023): “The use of non-nutritive sweeteners to replace sugar-sweetened products may reduce total calorie and carbohydrate intake as long as there is not a compensatory increase in energy intake from other sources. There is evidence that low- and no calorie sweetened beverages are a viable alternative to water” [105].
- -
- Diabetes UK Evidenced-based Nutrition Guidelines for the Prevention and Management of Diabetes (2019): “Non-nutritive sweeteners are safe and can be recommended” [106,107].
Even in the early stages of the disease, patients with T2DM have an increased risk of complications, with cardiovascular (CV) and renal impairment often being among the earliest cardio–renal manifestations. Cardiovascular and renal disease are major contributors to increased mortality in patients with T2DM [9,10,11,12,13,14,15,16,17]. The treatment of T2DM requires a timely, patient-centered, integrated and multifaceted approach that not only improves glycemic control and other risk factors but also reduces the risk of complications such as cardiovascular and renal disease [24]. The scientific evidence on fats and weight management focuses on the following points:
- Calorie intake: Many low-fat products contain added sugars to enhance flavor, which can lead to increased calorie consumption and, over time, weight gain.
- Satiety: Healthy fats (such as those found in avocados, nuts and olive oil) play a crucial role in reducing hunger pangs and making meals more filling. Without these fats, people can eat too many carbohydrates and proteins.
- Hormonal balance: Dietary fats support hormone production, including leptin, a hormone that regulates appetite and fat storage. A very-low-fat diet can disrupt this balance.
- Insulin sensitivity: When fats are replaced by refined carbohydrates, this can lead to blood sugar spikes and crashes, which promote fat storage rather than fat loss [108].
This review highlights the relationship between meal planning, the nutrient profile of the overall diet and glycemic parameters in adults with type 2 diabetes. This relationship is consistent with dietary goals, which include the following:
Maintain glycemic control, including insulin therapy if necessary, in accordance with the individualized nutrition plan and physical activity model.
- -
- Achieve blood pressure and lipid profile targets.
- -
- Ensure adequate energy intake to support healthy weight and metabolic function.
- -
- Treatment of comorbidities such as arterial hypertension, hyperlipidemia, chronic kidney disease, cardiovascular disease and screening for celiac disease.
- -
- Prevention of diabetes complications, both immediate (hyper- and hypoglycemia) and long-term (micro- and macrovascular epilogs).
- -
- Promoting general health through balanced and nutritious food choices.
- -
- Meeting individual nutritional needs, taking into account personal and cultural preferences, encouraging willingness to change and maintaining enjoyment of food without unnecessary restrictions [85].
Another important finding is the frequency of meal consumption. According to Papakonstantinou et al., it is more beneficial to eat smaller and more frequent meals [109]. Another important finding is that home-cooked meals have a positive effect on postprandial blood glucose levels [63] and may be a sustainable strategy with cost benefits. Finally, convenience foods could be a good choice for people with limited free time, even for T2DM patients, if they are prepared with healthy ingredients [67]. Snacks could also be a convenient option [61], provided they have the right nutritional composition, including carbohydrates and fiber [65]. Timing may also play a crucial role, as eating snacks in the morning may minimize the impact on blood glucose levels [69]. However, eating a traditional breakfast has been shown to impair glucose response [49]. Last but not least, freezing or refrigerating foods such as bread or rice can lower their glycemic index by altering the starch structure [73,76].
Future studies should include a larger number of participants. Conducting such studies with a larger population size would provide more information on the changes in health biomarkers associated with the consumption of these functional foods. The use of functional foods and branded foods with solid scientific evidence of efficacy could help reduce the incidence of diseases such as diabetes and dyslipidemia. This emphasizes the need for a well-designed diet consisting primarily of whole grains, fruits, vegetables and healthy fats, with the controlled addition of functional foods. Considering the principles of personalized nutrition for disease prevention, another crucial aspect to consider is that the effect of a food ingredient may vary from person to person. The future of metabolic care depends on a holistic approach where dietary carbohydrate restriction and ongoing remote support can safely help adults with T2DM to reduce HbA1c, weight and medication use [110,111,112,113,114].
5. Conclusions
In conclusion, the nutritional profile of most of the meals examined in this study is well in line with current recommendations for T2DM management. However, the American Diabetes Association’s Standards of Care 2025 states, “Data do not support a specific macronutrient pattern, and consideration is given to reducing total carbohydrate intake in adults with diabetes to improve blood glucose levels.” Healthy fats should be prioritized, including sources such as nuts, seeds, avocados and oily fish. The focus should be on eating unsaturated fats from sources such as nuts, seeds, avocados and oily fish, while processed foods and hidden sugars should be limited. Furthermore, the results suggest that the timing and frequency of meals are crucial for optimizing glucose metabolism. Eating larger meals early in the day may improve glycemic response, while frequent, smaller meals may further stabilize postprandial blood glucose levels. Preparing meals at home has been identified as a beneficial strategy to improve diet quality and glycemic outcomes, while premade meals and snacks, when composed of nutritious ingredients, may provide a convenient alternative for individuals with limited time. By reinforcing these dietary principles and prioritizing high-quality, nutrient-dense foods, people with T2DM can improve their metabolic health.
Author Contributions
Conceptualization, O.A., A.B., O.G., and M.D.; methodology, A.B., O.A., O.G., and M.D.; software, M.D.; validation, O.A., A.B., M.D., and M.K.; formal analysis, M.D.; investigation, O.A., M.D., and M.K.; resources, O.A., M.D., and M.K.; data curation, M.D.; writing—original draft preparation, M.D.; writing—review and editing, O.A., M.D., and M.K.; visualization, A.B., O.G., and O.A.; supervision, O.G., O.A., and A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created.
Conflicts of Interest
The authors declare no conflicts of interest. Author Olga Gortzi was employed by the company POSS—Driving Innovation in Functional Foods PCC. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Boels, A.M.; Rutten, G.; Zuithoff, N.; De Wit, A.; Vos, R. Effectiveness of diabetes self-management education via a smartphone application in insulin treated type 2 diabetes patients–design of a randomised controlled trial (‘TRIGGER study’). BMC Endocr. Disord. 2018, 18, 74. [Google Scholar] [CrossRef] [PubMed]
- Abdul Basith Khan, M.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of type 2 diabetes—Global burden of disease and forecasted trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef]
- Car, J.; Collaborators, G.D. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef] [PubMed]
- Moschonis, G.; Grammatikaki, E.; Manios, Y. Perinatal predictors of overweight at infancy and preschool childhood: The GENESIS study. Int. J. Obes. 2008, 32, 39–47. [Google Scholar] [CrossRef]
- Cardon, G.; De Bourdeaudhuij, I.; Iotova, V.; Latomme, J.; Socha, P.; Koletzko, B.; Moreno, L.; Manios, Y.; Androutsos, O.; De Craemer, M. Health related behaviours in normal weight and overweight preschoolers of a large pan-European sample: The ToyBox-study. PLoS ONE 2016, 11, e0150580. [Google Scholar] [CrossRef]
- Moschonis, G.; Siopis, G.; Anastasiou, C.; Iotova, V.; Stefanova, T.; Dimova, R.; Rurik, I.; Radó, A.S.; Cardon, G.; De Craemer, M. Prevalence of childhood obesity by country, family socio-demographics, and parental obesity in Europe: The Feel4Diabetes study. Nutrients 2022, 14, 1830. [Google Scholar] [CrossRef]
- Makrilakis, K.; Kalpourtzi, N.; Ioannidis, I.; Iraklianou, S.; Raptis, A.; Sotiropoulos, A.; Gavana, M.; Vantarakis, A.; Kantzanou, M.; Hadjichristodoulou, C. Prevalence of diabetes and pre-diabetes in Greece. Results of the First National Survey of Morbidity and Risk Factors (EMENO) study. Diabetes Res. Clin. Pract. 2021, 172, 108646. [Google Scholar] [CrossRef]
- Vassou, C.; Georgousopoulou, E.N.; Chrysohoou, C.; Yannakoulia, M.; Pitsavos, C.; Cropley, M.; Panagiotakos, D.B. Irrational beliefs trigger depression and anxiety symptoms, and associated with increased inflammation and oxidative stress markers in the 10-year diabetes mellitus risk: The ATTICA epidemiological study. J. Diabetes Metab. Disord. 2021, 20, 727–739. [Google Scholar] [CrossRef]
- Chen, Y.; Lee, K.; Ni, Z.; He, J.C. Diabetic kidney disease: Challenges, advances, and opportunities. Kidney Dis. 2020, 6, 215–225. [Google Scholar] [CrossRef]
- Hayden, M.R. Overview and new insights into the metabolic syndrome: Risk factors and emerging variables in the development of type 2 diabetes and cerebrocardiovascular disease. Medicina 2023, 59, 561. [Google Scholar] [CrossRef] [PubMed]
- Bugger, H.; Abel, E.D. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia 2014, 57, 660–671. [Google Scholar] [CrossRef]
- Tahraoui, A.; Israili, Z.H.; Lyoussi, B. Hypoglycemic and Hypolipidemic Effects of Aqueous Extracts of Ajuga iva and Centaurium erythreae on a Rodent Model of Metabolic Syndrome. Nat. Prod. J. 2017, 7, 47–57. [Google Scholar] [CrossRef]
- Fowler, M.J. Microvascular and macrovascular complications of diabetes. Clin. Diabetes 2011, 29, 116–122. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Banerji, M.; Bray, G.A.; Buchanan, T.A.; Clement, S.; Henry, R.R.; Kitabchi, A.E.; Mudaliar, S.; Musi, N.; Ratner, R. Actos Now for the prevention of diabetes (ACT NOW) study. BMC Endocr. Disord. 2009, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Marwick, T.H.; Ritchie, R.; Shaw, J.E.; Kaye, D. Implications of underlying mechanisms for the recognition and management of diabetic cardiomyopathy. J. Am. Coll. Cardiol. 2018, 71, 339–351. [Google Scholar] [CrossRef]
- Sposito, A.C.; Berwanger, O.; de Carvalho, L.S.F.; Saraiva, J.F.K. Correction to: GLP-1RAs in type 2 diabetes: Mechanisms that underlie cardiovascular effects and overview of cardiovascular outcome data. Cardiovasc. Diabetol. 2019, 18, 157. [Google Scholar] [CrossRef]
- Leon, B.M.; Maddox, T.M. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J. Diabetes 2015, 6, 1246. [Google Scholar] [CrossRef] [PubMed]
- Gortzi, O.; Dimopoulou, M.; Androutsos, O.; Vraka, A.; Gousia, H.; Bargiota, A. Effectiveness of a Nutrition Education Program for Patients with Type 2 Diabetes Mellitus. Appl. Sci. 2024, 14, 2114. [Google Scholar] [CrossRef]
- Yannakoulia, M.; Scarmeas, N. Diets. N. Engl. J. Med. 2024, 390, 2098–2106. [Google Scholar] [CrossRef]
- Alkhatib, A.; Tsang, C.; Tiss, A.; Bahorun, T.; Arefanian, H.; Barake, R.; Khadir, A.; Tuomilehto, J. Functional foods and lifestyle approaches for diabetes prevention and management. Nutrients 2017, 9, 1310. [Google Scholar] [CrossRef]
- DeFronzo, R.A. From the triumvirate to the ominous octet: A new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009, 58, 773–795. [Google Scholar] [CrossRef] [PubMed]
- Garvey, W.T.; Mechanick, J.I.; Einhorn, D. The American Association of Clinical Endocrinologists and the American College of Endocrinology: 2014 advanced framework for a new diagnosis of obesity as a chronic disease. Endocr. Pract. 2014, 20, 977. [Google Scholar] [CrossRef]
- EFSA, E. Dietary reference values for nutrients summary report. EFSA Support. Publ. 2017, 14, e15121E. [Google Scholar] [CrossRef]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022, 45, 2753–2786. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Itsiopoulos, C.; Mayr, H.L.; Thomas, C.J. The anti-inflammatory effects of a Mediterranean diet: A review. Curr. Opin. Clin. Nutr. Metab. Care 2022, 25, 415–422. [Google Scholar] [CrossRef]
- Hernáez, Á.; Castañer, O.; Elosua, R.; Pintó, X.; Estruch, R.; Salas-Salvadó, J.; Corella, D.; Arós, F.; Serra-Majem, L.; Fiol, M.; et al. Mediterranean Diet Improves High-Density Lipoprotein Function in High-Cardiovascular-Risk Individuals. Circulation 2017, 135, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Maiorino, M.I.; Bellastella, G.; Petrizzo, M.; Scappaticcio, L.; Giugliano, D.; Esposito, K. Mediterranean diet cools down the inflammatory milieu in type 2 diabetes: The MÉDITA randomized controlled trial. Endocrine 2016, 54, 634–641. [Google Scholar] [CrossRef]
- Shai, I.; Schwarzfuchs, D.; Henkin, Y.; Shahar, D.R.; Witkow, S.; Greenberg, I.; Golan, R.D.; Fraser, D.; Bolotin, A.; Vardi, H.; et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N. Engl. J. Med. 2008, 359, 229–241. [Google Scholar] [CrossRef]
- Kouvari, M.; Chrysohoou, C.; Damigou, E.; Barkas, F.; Kravvariti, E.; Liberopoulos, E.; Tsioufis, C.; Sfikakis, P.P.; Pitsavos, C.; Panagiotakos, D.; et al. Non-invasive tools for liver steatosis and steatohepatitis predict incidence of diabetes, cardiovascular disease and mortality 20 years later: The ATTICA cohort study (2002–2022). Clin. Nutr. 2024, 43, 900–908. [Google Scholar] [CrossRef]
- Bhanpuri, N.H.; Hallberg, S.J.; Williams, P.T.; McKenzie, A.L.; Ballard, K.D.; Campbell, W.W.; McCarter, J.P.; Phinney, S.D.; Volek, J.S. Cardiovascular disease risk factor responses to a type 2 diabetes care model including nutritional ketosis induced by sustained carbohydrate restriction at 1 year: An open label, non-randomized, controlled study. Cardiovasc. Diabetol. 2018, 17, 56. [Google Scholar] [CrossRef]
- Tay, J.; Luscombe-Marsh, N.D.; Thompson, C.H.; Noakes, M.; Buckley, J.D.; Wittert, G.A.; Yancy, W.S., Jr.; Brinkworth, G.D. Comparison of low-and high-carbohydrate diets for type 2 diabetes management: A randomized trial. Am. J. Clin. Nutr. 2015, 102, 780–790. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Huang, W.S.; Ho, M.H.; Chang, C.H.; Lee, L.T.; Chen, H.S.; Kang, Y.-D.; Chie, W.-C.; Jan, C.-F.; Wang, W.-D.; et al. The potential prolonged effect at one-year follow-up after 18-month randomized controlled trial of a 90 g/day low-carbohydrate diet in patients with type 2 diabetes. Nutr. Diabetes 2022, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Lasa, A.; Miranda, J.; Bulló, M.; Casas, R.; Salas-Salvadó, J.; Larretxi, I.; Estruch, R.; Ruiz-Gutiérrez, V.; Portillo, M.P. Comparative effect of two Mediterranean diets versus a low-fat diet on glycaemic control in individuals with type 2 diabetes. Eur. J. Clin. Nutr. 2014, 68, 767–772. [Google Scholar] [CrossRef]
- Esposito, K.; Maiorino, M.I.; Ciotola, M.; Di Palo, C.; Scognamiglio, P.; Gicchino, M.; Petrizzo, M.; Saccomanno, F.; Beneduce, F.; Ceriello, A.; et al. Effects of a Mediterranean-style diet on the need for antihyperglycemic drug therapy in patients with newly diagnosed type 2 diabetes: A randomized trial. Ann. Intern. Med. 2009, 151, 306–314. [Google Scholar] [CrossRef]
- Kavanagh, M.E.; Back, S.; Chen, V.; Glenn, A.J.; Viscardi, G.; Houshialsadat, Z.; Sievenpiper, J.L.; Kendall, C.W.C.; Jenkins, D.J.A.; Chiavaroli, L. The Portfolio Diet and HbA1c in Adults Living with Type 2 Diabetes Mellitus: A Patient-Level Pooled Analysis of Two Randomized Dietary Trials. Nutrients 2024, 16, 2817. [Google Scholar] [CrossRef]
- Carter, S.; Clifton, P.M.; Keogh, J.B. Effect of intermittent compared with continuous energy restricted diet on glycemic control in patients with type 2 diabetes: A randomized noninferiority trial. JAMA Netw. Open 2018, 1, e180756. [Google Scholar] [CrossRef] [PubMed]
- Krebs, J.D.; Elley, C.R.; Parry-Strong, A.; Lunt, H.; Drury, P.L.; Bell, D.A.; Robinson, E.; Moyes, S.A.; Mann, J.I. The Diabetes Excess Weight Loss (DEWL) Trial: A randomised controlled trial of high-protein versus high-carbohydrate diets over 2 years in type 2 diabetes. Diabetologia 2012, 55, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Barnard, N.D.; Cohen, J.; Jenkins, D.J.; Turner-McGrievy, G.; Gloede, L.; Green, A.; Ferdowsian, H. A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: A randomized, controlled, 74-wk clinical trial. Am. J. Clin. Nutr. 2009, 89, 1588S–1596S. [Google Scholar] [CrossRef]
- Yaikwawong, M.; Jansarikit, L.; Jirawatnotai, S.; Chuengsamarn, S. Curcumin extract improves beta cell functions in obese patients with type 2 diabetes: A randomized controlled trial. Nutr. J. 2024, 23, 119. [Google Scholar] [CrossRef] [PubMed]
- Lean, M.E.; Leslie, W.S.; Barnes, A.C.; Brosnahan, N.; Thom, G.; McCombie, L.; Kelly, Τ.; Irvine, K.; Peters, C.; Zhyzhneuskaya, S.; et al. 5-year follow-up of the randomised Diabetes Remission Clinical Trial (DiRECT) of continued support for weight loss maintenance in the UK: An extension study. Lancet Diabetes Endocrinol. 2024, 12, 233–246. [Google Scholar] [CrossRef]
- Kouvari, M.; Tsiampalis, T.; Damigou, E.; Barkas, F.; Chrysohoou, C.; Kravvariti, E.; Kassanis, Κ.; Dalmyras, D.; Anastasiou, G.; Koutsogianni, A.D.; et al. Investigating the diverse role of ultra-processed foods on cardiometabolic multimorbidity: Highlights from the ATTICA prospective study (2002–2022). Eur. Heart J. 2024, 45 (Suppl. 1), ehae666.2649. [Google Scholar] [CrossRef]
- Balfegó Díaz, M. Diabetis Mellitus Tipus 2: Impacte Metabòlic D’una Dieta Rica en Sardina. Ph.D. Thesis, Universitat de Barcelona, Barcelona, Spain, 2017. [Google Scholar]
- Nurdin, N.M.; Navratilova, H.F.; Ekawidyani, K.R.; Kurniawan, M.Y. Soy flour snack bars lower glycaemic response in type 2 diabetes mellitus subjects: A randomised cross-over design. Mal. J. Nutr. 2022, 28, 163–175. [Google Scholar] [CrossRef]
- Chaiyakul, S.; Ketkham, N.; Chaichana, C.; Khumkhana, N.; Deekum, W.; Wongshaya, P.; Suwanmalai, T.; Hutchinson, C.; Pramyothin, P. Effects of a novel rice-based diabetes-specific formula on postprandial glucose and gastrointestinal hormones: A double-blinded multi-arm randomized crossover trial. Front. Endocrinol. 2023, 14, 1141497. [Google Scholar] [CrossRef]
- Foshati, S.; Nouripour, F.; Akhlaghi, M. Effect of Date and Raisin Snacks on Glucose Response in Type 2 Diabetes. Nutr. Food Sci. Res. 2015, 2, 19–25. [Google Scholar]
- de Carvalho, C.M.; de Paula, T.P.; Viana, L.V.; Machado, V.M.; de Almeida, J.C.; Azevedo, M.J. Plasma glucose and insulin responses after consumption of breakfasts with different sources of soluble fiber in type 2 diabetes patients: A randomized crossover clinical trial. Am. J. Clin. Nutr. 2017, 106, 1238–1245. [Google Scholar] [CrossRef]
- Mustad, V.A.; Hegazi, R.A.; Hustead, D.S.; Budiman, E.S.; Rueda, R.; Maki, K.; Powers, M.; Mechanick, J.I.; Bergenstal, R.M.; Hamdy, O. Use of a diabetes-specific nutritional shake to replace a daily breakfast and afternoon snack improves glycemic responses assessed by continuous glucose monitoring in people with type 2 diabetes: A randomized clinical pilot study. BMJ Open Diabetes Res. Care 2020, 8, e001258. [Google Scholar] [CrossRef]
- Centofanti, S.; Dorrian, J.; Hilditch, C.; Grant, C.; Coates, A.; Banks, S. Eating on nightshift: A big vs small snack impairs glucose response to breakfast. Neurobiol. Sleep Circadian Rhythm. 2018, 4, 44–48. [Google Scholar] [CrossRef]
- Christiansen, C.B.; Jeppesen, P.B.; Hermansen, K.; Gregersen, S. Aronia in the Type 2 Diabetes Treatment Regimen. Nutrients 2023, 15, 4188. [Google Scholar] [CrossRef]
- Olagunju, A.I.; Arigbede, T.I.; Oyeleye, I.S.; Makanjuola, S.A.; Oyebode, E.T.; Enikuomehin, A.C. High-protein, low glycemic index snack from optimized blend of three wholegrains exhibits nutraceutical quality and elicits low glycemic response in diabetic human subjects. Food Prod. Process. Nut. 2024, 6, 32. [Google Scholar] [CrossRef]
- Bae, J.H.; Kim, L.K.; Min, S.H.; Ahn, C.H.; Cho, Y.M. Postprandial glucose-lowering effect of premeal consumption of protein-enriched, dietary fiber-fortified bar in individuals with type 2 diabetes mellitus or normal glucose tolerance. J. Diabetes Investig. 2018, 9, 1110–1118. [Google Scholar] [CrossRef]
- Nurdin, N.M.; Navratilova, H.F.; Ekawidyani, K.R.; Pratiwi, D.; Kurniawan, M.Y. Soy Flour-Based Snack Bar as Potential Snack Alternative for Diabetes Mellitus. J. Gizi Dan Pangan 2020, 15, 125–132. [Google Scholar] [CrossRef]
- Smith, T.J.; Karl, J.P.; Wilson, M.A.; Whitney, C.C.; Barrett, A.; Farhadi, N.F.; Chen, C.-Y.O.; Montain, S.J. Glycaemic regulation, appetite and ex vivo oxidative stress in young adults following consumption of high-carbohydrate cereal bars fortified with polyphenol-rich berries. Br. J. Nutr. 2019, 121, 1026–1038. [Google Scholar] [CrossRef]
- Yan, M.; Rush, E.; Jackson, R.; Shaikh, S. Snack (re) formulation in the improvement of health effects on glycaemia and satiety responses: Preliminary results. Food Nutr. Sci. 2020, 11, 649–658. [Google Scholar] [CrossRef]
- Gourineni, V.; Stewart, M.L.; Wilcox, M.L.; Maki, K.C. Nutritional bar with potato-based resistant starch attenuated post-prandial glucose and insulin response in healthy adults. Foods 2020, 9, 1679. [Google Scholar] [CrossRef] [PubMed]
- Wolever, T.M.; Van Klinken, B.J.-W.; Bordenave, N.; Kaczmarczyk, M.; Jenkins, A.L.; Chu, Y.; Harkness, L. Reformulating cereal bars: High resistant starch reduces in vitro digestibility but not in vivo glucose or insulin response; whey protein reduces glucose but disproportionately increases insulin. Am. J. Clin. Nutr. 2016, 104, 995–1003. [Google Scholar] [CrossRef]
- Evert, A.B.; Dennison, M.; Gardner, C.D.; Garvey, W.T.; Lau, K.H.K.; MacLeod, J.; Mitri, J.; Pereira, R.F.; Rawlings, K.; Robinson, S. Nutrition therapy for adults with diabetes or prediabetes: A consensus report. Diabetes Care 2019, 42, 731. [Google Scholar] [CrossRef] [PubMed]
- Reader, D.M.; O’Connell, B.S.; Johnson, M.L.; Franz, M. Glycemic and insulinemic response of subjects with type 2 diabetes after consumption of three energy bars. J. Acad. Nutr. Diet. 2002, 102, 1139. [Google Scholar] [CrossRef]
- Urita, Y.; Noda, T.; Watanabe, D.; Iwashita, S.; Hamada, K.; Sugimoto, M. Effects of a soybean nutrition bar on the postprandial blood glucose and lipid levels in patients with diabetes mellitus. Int. J. Food Sci. Nutr. 2012, 63, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulou, M.; Vareltzis, P.; Floros, S.; Androutsos, O.; Bargiota, A.; Gortzi, O. Postprandial Glucose Response in Type 2 Diabetes Mellitus Patients and Possible Antioxidant Properties of a Plant-Based Snack Bar. Foods 2024, 13, 4123. [Google Scholar] [CrossRef]
- Cai, J.-S.; Feng, J.-Y.; Ni, Z.-J.; Ma, R.-H.; Thakur, K.; Wang, S.; Hu, F.; Zhang, J.-G.; Wei, Z.-J. An update on the nutritional, functional, sensory characteristics of soy products, and applications of new processing strategies. Trends Food Sci. Technol. 2021, 112, 676–689. [Google Scholar] [CrossRef]
- Fatoumata, C.; Wahauwouele Hermann, C.; Tano Marie-Ange Sakia, M.; Koua Jean-Brice, A.; Salimata, C.; Vijayakumar, V.; Camelia, D.; Florentina, M. Household Production of Cookies from Sorghum (Sorghum bicolor) with a Low Glycaemic Index in Prevention and Management of Type 2 Diabetes in Côte d’Ivoire. Curr. Nutr. Food Sci. 2025, 21, 341–349. [Google Scholar] [CrossRef]
- Sasani, N.; Kazemi, A.; Babajafari, S.; Amiri-Ardekani, E.; Rezaiyan, M.; Barati-Boldaji, R.; Mazloomi, S.M.; Clark, C.C.; Ashrafi-Dehkordi, E. The effect of acorn muffin consumption on glycemic indices and lipid profile in type 2 diabetic patients: A randomized double-blind placebo-controlled clinical trial. Food Sci. Nutr. 2023, 11, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulou, M.; Vareltzis, P.; Floros, S.; Androutsos, O.; Bargiota, A.; Gortzi, O. Development of a Functional Acceptable Diabetic and Plant-Based Snack Bar Using Mushroom (Coprinus comatus) Powder. Foods 2023, 12, 2702. [Google Scholar] [CrossRef] [PubMed]
- Eliana, F.; Pranoto, B.A. A randomized controlled clinical trial of carbohydrate mix-fortified nutrition in type 2 diabetes mellitus patients. Med. J. Indones. 2020, 29, 275–282. [Google Scholar] [CrossRef]
- Manios, Y.; Moschonis, G.; Mavrogianni, C.; Tsoutsoulopoulou, K.; Kogkas, S.; Lambrinou, C.-P.; Efstathopoulou, E. Postprandial glucose and insulin levels in type 2 diabetes mellitus patients after consumption of ready-to-eat mixed meals. Eur. J. Nutr. 2017, 56, 1359–1367. [Google Scholar] [CrossRef]
- Apergi, K.; Karatzi, K.; Reppas, K.; Karaglani, E.; Usheva, N.; Giménez-Legarre, N.; Moreno, L.A.; Dimova, R.; Antal, E.; Jemina, K.; et al. Association of breakfast consumption frequency with fasting glucose and insulin sensitivity/b cells function (HOMA-IR) in adults from high-risk families for type 2 diabetes in Europe: The Feel4Diabetes Study. Eur. J. Clin. Nutr. 2022, 76, 1600–1610. [Google Scholar] [CrossRef]
- Vujović, N.; Piron, M.J.; Qian, J.; Chellappa, S.L.; Nedeltcheva, A.; Barr, D.; Heng, S.W.; Kerlin, K.; Srivastav, S.; Wang, W.; et al. Late isocaloric eating increases hunger, decreases energy expenditure, and modifies metabolic pathways in adults with overweight and obesity. Cell Metab. 2022, 34, 1486–1498.e7. [Google Scholar] [CrossRef]
- Putriani, N.; Perdana, J.; Meiliana; Nugrahedi, P.Y. Effect of thermal processing on key phytochemical compounds in green leafy vegetables: A review. Food Rev. Int. 2022, 38, 783–811. [Google Scholar] [CrossRef]
- Bao, S.; Li, X.; Lan, T.; Wang, J.; Hu, Y.; Sun, X.; Ma, T. Effects of different cooking treatments on the sensory qualities and pigmented phytochemicals of carrots. Food Chem. 2023, 405, 135015. [Google Scholar] [CrossRef]
- Cheng, J.; Li, J.; Xiong, R.-G.; Wu, S.-X.; Xu, X.-Y.; Tang, G.-Y.; Huang, S.-Y.; Zhou, D.-D.; Li, H.; Feng, Y. Effects and mechanisms of anti-diabetic dietary natural products: An updated review. Food Funct. 2024, 15, 1758–1778. [Google Scholar] [CrossRef] [PubMed]
- Guiné, R. The drying of foods and its effect on the physical-chemical, sensorial and nutritional properties. Int. J. Food Eng. 2018, 2, 93–100. [Google Scholar] [CrossRef]
- Arvaniti, O.S.; Samaras, Y.; Gatidou, G.; Thomaidis, N.S.; Stasinakis, A.S. Review on fresh and dried figs: Chemical analysis and occurrence of phytochemical compounds, antioxidant capacity and health effects. Food Res. Int. 2019, 119, 244–267. [Google Scholar] [CrossRef]
- Omolola, A.O.; Jideani, A.I.; Kapila, P.F. Quality properties of fruits as affected by drying operation. Crit. Rev. Food Sci. Nutr. 2017, 57, 95–108. [Google Scholar] [CrossRef]
- Borczak, B.; Sikora, M.; Sikora, E.; Dobosz, A.; Kapusta-Duch, J. Glycaemic index of wheat bread. Starch-Stärke 2018, 70, 1700022. [Google Scholar] [CrossRef]
- Skřivan, P.; Sluková, M.; Sinica, A.; Bleha, R.; Švec, I.; Šárka, E.; Pourová, V. Glycaemic Index of Bakery Products and Possibilities of Its Optimization. Appl. Sci. 2024, 14, 6070. [Google Scholar] [CrossRef]
- Cediel, G.; Reyes, M.; da Costa Louzada, M.L.; Steele, E.M.; Monteiro, C.A.; Corvalán, C.; Uauy, R. Ultra-processed foods and added sugars in the Chilean diet (2010). Public Health Nutr. 2018, 21, 125–133. [Google Scholar] [CrossRef]
- Coe, S.; Spiro, A. Cooking at home to retain nutritional quality and minimise nutrient losses: A focus on vegetables, potatoes and pulses. Nutr. Bull. 2022, 47, 538–562. [Google Scholar] [CrossRef]
- Rajpurohit, B.; Li, Y. Overview on pulse proteins for future foods: Ingredient development and novel applications. J. Future Foods 2023, 3, 340–356. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies. Scientific opinion on dietary reference values for carbohydrates and dietary fibre. EFSA J. 2010, 8, 1462. [Google Scholar] [CrossRef]
- Delcour, J.A.; Aman, P.; Courtin, C.M.; Hamaker, B.R.; Verbeke, K. Prebiotics, Fermentable Dietary Fiber, and Health Claims. Adv. Nutr. 2016, 7, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, A.; Tsang, C.; Tuomilehto, J. Olive oil nutraceuticals in the prevention and management of diabetes: From molecules to lifestyle. Int. J. Mol. Sci. 2018, 19, 2024. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.; McArdle, P.; Taplin, J.; Unwin, D.; Unwin, J.; Deakin, T.; Wheatley, S.; Murdoch, C.; Malhotra, A.; Mellor, D. Dietary strategies for remission of type 2 diabetes: A narrative review. J. Hum. Nutr Diet. 2022, 35, 165–178. [Google Scholar] [CrossRef]
- The Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes (EASD). Evidence-based European recommendations for the dietary management of diabetes. Diabetologia 2023, 66, 965–985. [Google Scholar] [CrossRef]
- Ewers, B.; Blond, M.B.; Bruun, J.M.; Vilsbøll, T. Effects of basic carbohydrate counting versus standard dietary care for glycaemic control in type 2 diabetes (The BCC Study): A randomised, controlled trial. Nutr. Diabetes 2024, 14, 47. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 1. Improving Care and Promoting Health in Populations: Standards of Care in Diabetes—2025. Diabetes Care 2025, 48 (Suppl. 1), S14–S26. [Google Scholar] [CrossRef] [PubMed]
- Sasson, C.; Eckel, R.; Alger, H.; Bozkurt, B.; Carson, A.; Daviglus, M.; Deedwania, P.; Kirley, K.; Lamendola, C.; Nguyen, M.; et al. American heart association diabetes and cardiometabolic health summit: Summary and recommendations. J. Am. Heart Assoc. 2018, 7, e009271. [Google Scholar] [CrossRef]
- Koloverou, E.; Panagiotakos, D.B. Macronutrient composition and management of non-insulin-dependent diabetes mellitus (NIDDM): A new paradigm for individualized nutritional therapy in diabetes patients. Rev. Diabet. Stud. RDS 2016, 13, 6. [Google Scholar] [CrossRef]
- Steinberg, D.; Kay, M.; Burroughs, J.; Svetkey, L.P.; Bennett, G.G. The effect of a digital behavioral weight loss intervention on adherence to the Dietary Approaches to Stop Hypertension (DASH) dietary pattern in medically vulnerable primary care patients: Results from a randomized controlled trial. J. Acad. Nutr. Diet. 2019, 119, 574–584. [Google Scholar] [CrossRef]
- Patel, L.; Alicandro, G.; La Vecchia, C. Dietary Approaches to Stop Hypertension (DASH) diet and associated socio-economic inequalities in the UK. Br. J. Nutr. 2020, 124, 1076–1085. [Google Scholar] [CrossRef]
- Papamichou, D.; Panagiotakos, D.B.; Itsiopoulos, C. Dietary patterns and management of type 2 diabetes: A systematic review of randomised clinical trials. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 531–543. [Google Scholar] [CrossRef]
- Manios, Y.; Grammatikaki, E.; Papoutsou, S.; Liarigkovinos, T.; Kondaki, K.; Moschonis, G. Nutrient intakes of toddlers and preschoolers in Greece: The GENESIS study. J. Am. Diet. Assoc. 2008, 108, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Miguel-Berges, M.L.; Mouratidou, T.; Santaliestra-Pasias, A.; Androutsos, O.; Iotova, V.; Galcheva, S.; De Craemer, M.; Cardon, G.; Koletzko, B.; Kulaga, Z.; et al. Longitudinal associations between diet quality, sedentary behaviours and physical activity and risk of overweight and obesity in preschool children: The ToyBox-study. Pediatr. Obes. 2023, 18, e13068. [Google Scholar] [CrossRef]
- Manios, Y.; Kourlaba, G.; Kondaki, K.; Grammatikaki, E.; Anastasiadou, A.; Roma-Giannikou, E. Obesity and television watching in preschoolers in Greece: The GENESIS study. Obesity 2009, 17, 2047–2053. [Google Scholar] [CrossRef]
- Manios, Y.; Grammatikaki, E.; Kondaki, K.; Ioannou, E.; Anastasiadou, A.; Birbilis, M. The effect of maternal obesity on initiation and duration of breast-feeding in Greece: The GENESIS study. Public Health Νutr. 2009, 12, 517–524. [Google Scholar] [CrossRef]
- Manios, Y.; Kondaki, K.; Kourlaba, G.; Grammatikaki, E.; Birbilis, M.; Ioannou, E. Television viewing and food habits in toddlers and preschoolers in Greece: The GENESIS study. Eur. J. Pediatr. 2009, 168, 801–808. [Google Scholar] [CrossRef]
- Manios, Y.; Kourlaba, G.; Kondaki, K.; Grammatikaki, E.; Birbilis, M.; Oikonomou, E.; Roma-Giannikou, E. Diet Quality of Preschoolers in Greece Based on the Healthy Eating Index: The GENESIS Study. J. Am. Diet. Assoc. 2009, 109, 616–623. [Google Scholar] [CrossRef]
- Pinket, A.-S.; De Craemer, M.; Huybrechts, I.; De Bourdeaudhuij, I.; Deforche, B.; Cardon, G.; Androutsos, O.; Koletzko, B.; Moreno, L.A.; Socha, P.; et al. Multibehavioural Interventions with a Focus on Specific Energy Balance-Related Behaviours Can Affect Diet Quality in Preschoolers from Six European Countries: The ToyBox-Study. Nutrients 2017, 9, 479. [Google Scholar] [CrossRef] [PubMed]
- Androutsos, O.; Saltaouras, G.; Kipouros, M.; Koutsaki, M.; Migdanis, A.; Georgiou, C.; Perperidi, M.; Papadopoulou, S.K.; Kosti, R.I.; Giaginis, C. Comparative Analysis of Dietary Behavior in Children and Parents During COVID-19 Lockdowns in Greece: Insights from a Non-Representative Sample. Nutrients 2024, 17, 112. [Google Scholar] [CrossRef]
- Rahimi, G.R.M.; Yousefabadi, H.A.; Niyazi, A.; Rahimi, N.M.; Alikhajeh, Y. Effects of Lifestyle Intervention on Inflammatory Markers and Waist Circumference in Overweight/Obese Adults With Metabolic Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Biol. Res. Nurs. 2022, 24, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Siró, I.; Kápolna, E.; Kápolna, B.; Lugasi, A. Functional food. Product development, marketing and consumer acceptance—A review. Appetite 2008, 51, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulou, M.; Vareltzis, P.; Gortzi, O. A Systematic Review of the Twelve Most Popular Bean Varieties, Highlighting Their Potential as Functional Foods Based on the Health Benefits Derived from Their Nutritional Profiles, Focused on Non-Communicable Diseases. Appl. Sci. 2024, 14, 10215. [Google Scholar] [CrossRef]
- Mann, J.; De Leeuw, I.; Hermansen, K.; Karamanos, B.; Karlström, B.; Katsilambros, N.; Riccardi, G.; Rivellese, A.; Rizkalla, S.; Slama, G. Diabetes and Nutrition Study Group (DNSG) of the European Association. Evidence-based nutritional approaches to the treatment and prevention of diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 2004, 14, 373–394. [Google Scholar] [CrossRef]
- ElSayed, N.A.; McCoy, R.G.; Aleppo, G.; Balapattabi, K.; Beverly, E.A.; Briggs Early, K.; Bruemmer, D.; Echouffo-Tcheugui, J.B.; Ekhlaspour, L.; Garg, R.; et al. 13. Older Adults: Standards of Care in Diabetes—2025. Diabetes Care 2025, 48, S266–S282. [Google Scholar]
- Bhattacharya, S.; Kalra, S. ADA–EASD Consensus Report on the Management of Hyperglycaemia in Type 2 Diabetes in an Afro-Asian Context: Broadening the Perspective. TouchREVIEWS Endocrinol. 2023, 19, 4. [Google Scholar] [CrossRef]
- Dyson, P.A. A practical guide to delivering nutritional advice to people with diabetes. Diabetes Ther. 2019, 10, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Castro-Barquero, S.; Ruiz-León, A.M.; Sierra-Pérez, M.; Estruch, R.; Casas, R. Dietary strategies for metabolic syndrome: A comprehensive review. Nutrients 2020, 12, 2983. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Kontogianni, M.; Mitrou, P.; Magriplis, E.; Vassiliadi, D.; Nomikos, T.; Lambadiari, V.; Georgousopoulou, E.; Dimitriadis, G. Effects of 6 vs 3 eucaloric meal patterns on glycaemic control and satiety in people with impaired glucose tolerance or overt type 2 diabetes: A randomized trial. Diabetes Metab. 2018, 44, 226–234. [Google Scholar] [CrossRef]
- Hallberg, S.J.; McKenzie, A.L.; Williams, P.T.; Bhanpuri, N.H.; Peters, A.L.; Campbell, W.W.; Hazbun, T.L.; Volk, B.M.; McCarter James, P.; Phinney Stephen, D.; et al. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: An open-label, non-randomized, controlled study. Diabetes Ther. 2018, 9, 583–612. [Google Scholar] [CrossRef]
- Davison, N.B.; Gaffney, C.J.; Kerns, J.G.; Zhuang, Q.D. Recent progress and perspectives on non-invasive glucose sensors. Diabetology 2022, 3, 56–71. [Google Scholar] [CrossRef]
- Tatti, P.; Pavandeep, S. Gender difference in type 1 diabetes: An underevaluated dimension of the disease. Diabetology 2022, 3, 364–368. [Google Scholar] [CrossRef]
- Sharma, M.; Vanam, H.P.; Panda, N.K.; Patro, S.K.; Arora, R.; Bhadada, S.K.; Rudramurthy, S.M.; Singh, M.P.; Koppula, P.R. Deciphering the neurosensory olfactory pathway and associated neo-immunometabolic vulnerabilities implicated in COVID-associated mucormycosis (CAM) and COVID-19 in a diabetes backdrop—A novel perspective. Diabetology 2022, 3, 193–235. [Google Scholar] [CrossRef]
- Brink, S.J. Insulin past, present, and future: 100 years from Leonard Thompson. Diabetology 2022, 3, 117–158. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).