Abstract
Background/Objectives: 5-Aminolevulinic acid (5-ALA) is a biosynthetic precursor of heme that induces heme oxygenase-1 (HO-1). Therapeutic induction of HO-1 has shown effectiveness in various autoimmune disease models, including type 1 diabetes (T1D). However, the efficacy of 5-ALA as an HO-1 inducer in T1D models remains unexplored. This study aimed to investigate the therapeutic efficacy of oral 5-ALA administration in preventing autoimmune diabetes development in nonobese diabetic (NOD) mice. Methods: We evaluated diabetes incidence, levels of insulin autoantibody, and severity of insulitis in 5-ALA-treated and control NOD mice. HO-1 expression of dendritic cells in the pancreatic islets and spleen of 5-ALA-treated NOD mice was measured. The IFN-γ/IL-17 of islet-infiltrating T cells and IL-10/IL-12 productions of dendritic cells in the spleen of 5-ALA-treated NOD mice were assessed. We stimulated islet antigen-specific CD4+ T cells with islet antigen-pulsed dendritic cells in the presence of 5-ALA and examined the proliferation of the T cells. Finally, we adoptively transferred islet antigen-specific CD4+ T cells into 5-ALA-treated, immunodeficient NOD-Rag1 knockout mice, and diabetes incidence in recipients was determined. Results: Oral 5-ALA treatment did not significantly impact diabetes incidence, levels of insulin autoantibody, and insulitis. No significant difference was observed in HO-1 expression in dendritic cells and cytokine production of T cells and dendritic cells. Similarly, there was no significant difference in the proliferation of islet antigen-specific CD4+ T cells in vitro and diabetes induction in transfer experiments. Conclusions: Oral administration of 5-ALA has a limited effect on suppressing the development of autoimmune diabetes in NOD mice.
1. Introduction
Type 1 diabetes (T1D) is an autoimmune disease where islet antigen-specific T cells are activated and proliferate in the islets, leading to the destruction of pancreatic beta cells [1]. Regulating these T cells is crucial for preventing the development and progression of T1D [2]. Studies on the prevention of T1D in humans have shown that immunomodulatory therapy targeting T cells, such as teplizumab, can transiently preserve beta cell function in high-risk individuals [3] and those with early-stage disease [4]. However, this treatment alone has not been able to completely prevent the disease. Therefore, combining immunotherapy with a safe and long-term adjunctive therapy may offer a more effective approach. 5-Aminolevulinic acid (5-ALA), the focus of this study, is a naturally occurring amino acid biosynthesized in vivo from glycine and succinyl-CoA in mitochondria [5]. In Japan, 5-ALA is widely marketed as a health supplement due to its ability to moderate postprandial blood glucose levels in individuals with impaired glucose tolerance [6]. Additionally, 5-ALA serves as a precursor for heme biosynthesis in vivo. When combined with sodium ferrous citrate (SFC), 5-ALA has been shown to enhance heme synthesis, phosphorylate mitogen-activated protein kinase, and induce the expression of heme oxygenase-1 (HO-1), known for its anti-inflammatory properties [7,8]. Interestingly, HO-1 expression in dendritic cells has been found to inhibit cytokine production, promote a tolerogenic phenotype, and suppress the maturation of dendritic cells [9,10,11,12,13]. Furthermore, HO-1 expression has been shown to suppress the differentiation and proliferation of inflammatory T-cell subsets, such as Th1 and Th17 cells, by downregulating costimulatory factors and enhancing the suppressive activity of regulatory T cells [14,15]. In the field of autoimmunity, HO-1 inducers have been used in various models of T-cell-driven diseases, such as rheumatoid arthritis, multiple sclerosis, and inflammatory bowel disease, and have been shown to suppress disease activity [16,17,18,19]. In the T1D model, systemic transduction of HO-1 in nonobese diabetic (NOD) mice suppressed insulitis and delayed the onset of diabetes [20]. Another study demonstrated that the upregulation of HO-1 with cobalt protoporphyrin IX (CoPP), an HO-1 inducer, reduced the number of dendritic cells infiltrating the pancreatic islets and suppressed insulitis [21]. Furthermore, coadministration of autoantigen and CoPP via intradermal injection reduced the migration of islet antigen-specific cytotoxic T cells to the islets [22]. Therefore, the potential of a harmless HO-1 inducer, 5-ALA, has raised interest in its therapeutic use in modulating immune activation and inducing tolerance in autoimmune diabetes.
To determine whether oral administration of 5-ALA can improve the immunopathogenesis of T1D and to identify the optimal dosage and timing of this treatment, the cumulative incidence of diabetes, insulitis levels, and insulin autoantibody (IAA) production in NOD mice treated with varying dosages and time courses of 5-ALA/SFC were assessed in this study. Additionally, we investigated the impact of 5-ALA on HO-1 expression and cytokine production in immune cells responsible for autoimmune diabetes, and proliferative and diabetogenic activity of islet antigen-specific CD4+ T cells through in vitro proliferation assay and adoptive transfer experiments.
2. Materials and Methods
2.1. Animals and Experimental Procedures
NOD mice, NOD.Cg-Tg (TcraBDC2.5, TcrbBDC2.5)/DoiJ (BDC2.5-NOD) mice, and NOD.129S7 (B6)-Rag1tm1Mom/J (Rag1KO-NOD) mice were bred and housed under pathogen-free conditions in the Research Center for Biomedical Models and Animal Welfare at Nagasaki University. BDC2.5-NOD and Rag1KO-NOD mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). All mice were maintained on a 12 h light/dark cycle and fed standard rodent food. Only female mice were used in this study. The same litter of mice was used for each individual experimental unit. Standardized processes were implemented for all cages, including feeding, watering, and handling, to reduce inconsistency across locations. Blinding was applied to animal care staff to ensure consistent care across all animals, regardless of their treatment group. Mice that met the predetermined criteria for sex and age were randomly assigned to groups. The analysis included all the animals, experimental units, and data points. All animal experiments were approved by the Institutional Animal Experimentation Committee and conducted following the Guidelines for Animal Experimentation.
2.2. Administration of 5-ALA
5-ALA and SFC (KIYAN PHARMA Co., Ltd., Tokyo, Japan) were dissolved in distilled water at a molar ratio of 1:0.5 immediately before administration. The solution was prepared and administered under light-shielded conditions. 5-ALA/SFC was given in drinking water at doses of 100 mg/kg or 300 mg/kg. The previous reports, which documented the effectiveness of oral 5-ALA administration in drinking water for other strains, were referred to determine the 5-ALA/SFC dosages [23,24,25]. The dilution concentration was calculated based on body weight and water intake data for each week of age from the same strain in our laboratory.
2.3. Monitoring the Development of Spontaneous Diabetes
To determine the optimal timing for administering 5-ALA, the NOD mice were given 100 mg/kg/day of 5-ALA/SFC from 4 to 12 weeks of age in Course 1, from 12 to 20 weeks of age in Course 2, from 4 to 20 weeks of age in Course 3, and the incidence of diabetes in each 5-ALA-treated group was compared to the control group. To evaluate the appropriate dosages of 5-ALA administration, the mice were divided into a standard-dose group (100 mg/kg), a high-dose group (300 mg/kg), and a control group. The 5-ALA-treated group received oral administration of 5-ALA from 4 to 50 weeks of age throughout the observation period. Blood glucose levels in mice were monitored for spontaneous diabetes using the OneTouch VerioVue blood glucose monitoring system (LifeScan, Tokyo, Japan) weekly from 12 to 50 weeks of age. Mice with blood glucose levels exceeding 250 mg/dL for two consecutive measurements were considered diabetic.
2.4. Measurement of Insulin Autoantibodies
Blood was collected from the tail vein of 12-week-old NOD mice that had been administered 5-ALA at doses of 100 mg/kg or 300 mg/kg from 4 weeks of age, and the control NOD mice. Insulin autoantibodies were measured using an enzyme-linked immunosorbent assay with 96-well plates, as previously described [26]. A noncompetitive assay was adopted, and samples were tested in duplicate with or without plate-bound human insulin. In brief, an ELISA plate (BioLegend, San Diego, CA, USA) was coated with or without 10 μg/mL of human insulin (Actrapid, Novo Nordisk, Bagsvaerd, Denmark) overnight at 4 °C. The wells were then blocked with PBS containing 2% BSA for 2 h at room temperature, after which they were probed with sera from the control and 5-ALA-treated mice (100 mg/kg or 300 mg/kg) at 12 weeks of age for 2 h (at a dilution of 1:10). The wells were washed four times, after which a biotinylated anti-mouse IgG1 antibody (Abcam, 1:10,000 dilution) was added for 30 min. After washing, horseradish peroxidase (HRP)-conjugated streptavidin (BioLegend, San Diego, CA, USA) was added for 15 min. After washing the plate five times, 3,3′,5,5′-tetramethylbenzidine (TMB) substrate solution (BioLegend, San Diego, CA, USA) was added, and the absorbance was measured at 450 nm using a Multiskan FC (Thermo Fisher Scientific, Waltham, MA, USA) microplate reader. Each sample was analyzed in duplicate, and the absorbance value at 450 nm was calculated by subtracting the absorbance of the sample without plate-bound insulin from the absorbance of the sample with plate-bound insulin.
2.5. Histology
Pancreatic sections from 12-week-old NOD mice that had been administered 5-ALA at doses of 100 mg/kg or 300 mg/kg from 4 weeks of age, and the control NOD mice, were histologically analyzed by hematoxylin and eosin and immunohistochemistry (IHC) staining of paraffin-embedded samples. Pancreata were fixed in 10% neutral buffered formalin. Pancreatic sections from three levels (200 µm apart) of paraffin-embedded samples were analyzed using hematoxylin and eosin staining. The severity of insulitis was blindly assessed by two independent investigators, and lymphocyte infiltration was scored in accordance with previous report [27] as follows: 0 (no lymphocytic infiltration), 1 (lymphocytic infiltration occupying < 25% of the total islet cell area), 2 (lymphocytic infiltration occupying 25–50% of the total islet cell area), 3 (lymphocytic infiltration occupying 50–75% of the total islet cell area), and 4 (lymphocytic infiltration occupying > 75% of the total islet cell area or small retracted islets). To prepare specimens for IHC staining for evaluating insulin expression, deparaffinized 4 μm sections were pretreated by heating in a microwave in Target Retrieval Solution (pH 9.0; Agilent Technologies, Santa Clara, CA, USA) for 20 min. The sections were then incubated with rabbit monoclonal anti-insulin antibody (1:1000; ab181547, Abcam, Cambridge, UK) for 1 h at 25 °C and then with goat antirabbit IgG (H&L) with horseradish peroxidase (1:2000; Abcam, ab205718, Cambridge, UK) for 1 h. All samples were further incubated with 3,3-diaminobenzidine hydrogen peroxide for 10 min and counterstained with hematoxylin. The insulin-positive area was calculated as a percentage of the total islet area within a field using BZ-X710 imaging software (ver. 1.4.1.1) (Keyence, Osaka, Japan).
2.6. Islet Isolation
Islets were isolated using collagenase P (Roche, Basel, Switzerland) and Histopaque-1077 density gradients (Sigma-Aldrich, St. Louis, MO, USA) [28]. In brief, the common bile duct was cannulated, and the pancreas was distended with 3 mL of HBSS containing 0.3 mg/mL collagenase P (Roche Diagnostics, Rotkreuz, Switzerland). The pancreata were then digested at 37 °C for 15 min and disrupted by shaking. The islets were then purified using a Histopaque-1077 density gradient (Sigma-Aldrich, St. Louis, MO, USA), washed, and harvested. For flow cytometry, the harvested islets were dispersed into single cells by a brief incubation in a solution of 0.011% trypsin (Thermo Fisher Scientific, Waltham, MA, USA) and 2 mM EDTA. The cells were then cultured for 1 h at 37 °C in 5% CO2 in RPMI (Thermo Fisher Scientific, Waltham, MA, USA) containing antibiotics, glutamine, and 10% FCS.
2.7. Flow Cytometry
The non-diabetic NOD mice over 12 weeks of age were used for flow cytometric analysis, and 5-ALA-treated mice received 1 week of oral administration of 5-ALA at a dosage of 100 mg/kg prior to the experiments. Fluorochrome-conjugated antibodies directed against the following antigens were used for flow cytometry: CD45 (30-F11), CD45.1 (A20), CD3ε (500A2), CD3ε (145-2C11), CD4 (GK1.5), CD8a (53–6.7), CD19 (6D5), CD11c (N418), CD11b (M1/70), F4/80 (BM8), HO-1 (HO-1-1), IFN-γ (XMG1.2), IL-17 (TC11-18H10), IL-10 (JES5–16E3), and IL-12 (C15.6). These antibodies were purchased from BioLegend (San Diego, CA, USA), BD Biosciences (Franklin Lakes, NJ, USA), and Thermo Fisher Scientific (Waltham, MA, USA). For HO-1 staining, intracellular staining with antibody to HO-1 was performed with the Intracellular Fixation/Permeabilization kit (Thermo Fisher Scientific, Waltham, MA, USA). The fixed cells were stained with primary unconjugated mouse anti-mouse HO-1 antibody (HO-1-1; MOPC-21, for isotype control) and stained with a secondary phycoerythrin-conjugated rat anti-mouse IgG1 (A85-1). For intracellular cytokine staining of IFN-γ and IL-17 among CD4+ T cells infiltrating into the islets, 2.0 × 105 cells per well underwent 3 h of stimulation with phorbol 12-myristate 13-acetate (PMA; 25 ng/mL) and ionomycin (1 µg/mL) plus brefeldin. The cells were first stained with anti-CD3ε and anti-CD4, and then stained intracellularly with anti-IL-17 and anti-IFN-γ, along with their respective isotype controls. Intracellular cytokine staining for activated dendritic cells was performed as described previously [29]. Dendritic cells were magnetically purified using CD11c-Selection Kits (StemCell Technologies, BC, Canada). A total of 2.0 × 105 dendritic cells per well were stimulated with the Toll-like receptor (TLR) 7/8 agonist R848 (Adipogen, San Diego, CA, USA) plus brefeldin for 4 h at a concentration of 1 μg/mL. The cells were harvested and stained with anti-CD11c, followed by intracellular staining with anti-IL-12, anti-IL-10, and their isotype controls. The Intracellular Fixation/Permeabilization kit (Thermo Fisher Scientific, Waltham, MA, USA) was used for staining these cytokines, and dead cells were excluded by the addition of either 7-aminoactinomycin D or Ghost Dye. All analyses were performed using the LSRFortessa™ (BD Biosciences) and FlowJo™ software (ver. 10.10.0) (BD Life Sciences, Franklin Lakes, NJ, USA).
2.8. Adoptive Transfer Experiments
Islet antigen-specific naïve CD4+ (BDC2.5) T cells were purified from the spleens of 8- to 12-week-old BDC2.5-NOD mice using naïve CD4+ T-cell Isolation Kit (StemCell Technologies, Vancouver, BC, Canada) in accordance with the manufacturer’s instructions. The purified naïve BDC2.5 CD4+ T cells were resuspended in EasySep™ Buffer (Stem Cell Technologies, Vancouver, BC, Canada) at 5.0 × 103 cells per 500 μL and intravenously injected into the tail veins of 8-week-old Rag1KO-NOD mice treated with 5-ALA from 4 weeks of age during the observation period. The mice were monitored for blood glucose daily until 19 days after the adoptive transfer. Blood glucose levels were measured twice per week thereafter.
2.9. Cell Culture and Carboxyfluorescein Succinimidyl Ester (CFSE) Labeling
In vitro stimulation of CD4+ T cells was performed as described previously [29]. Briefly, CD4+ T cells from BDC2.5 NOD mice (BDC2.5 CD4+ T cells) and dendritic cells from nondiabetic female NOD mice were purified from splenocytes using CD4+ T Cell-Isolation and CD11c-Selection Kits (StemCell Technologies, Vancouver, BC, Canada), respectively. Firstly, 1.0 × 104 dendritic cells per well were pulsed with the mimotope peptide, RLGL-WE14 (50 ng/mL, sequence: RLGLWSRMDQLAKELTAE) [30], in a 96-well round-bottomed plate for 1 h, in the presence of 5-ALA (500, 100, and 20 μM). Secondly, the BDC2.5 CD4+ T cells were labelled with CFSE in accordance with the manufacturer’s protocol (Tonbo Biosciences, San Diego, CA, USA). 1.0 × 105 CFSE-labeled BDC2.5 CD4+ T cells per well were cocultured with 1.0 × 104 RLGL-WE14-pulsed dendritic cells per well in a 96-well round-bottomed plate, in the presence of 5-ALA at concentrations of 500, 100, and 20 μM. The proliferation of the CD4+ T cells was assessed by flow cytometry after 4 days of incubation at 37 °C in 5% CO2.
2.10. Statistical Analysis
All statistical analyses were performed using GraphPad Prism software versions 9 and 10 (GraphPad, Boston, CA, USA). The unpaired Student’s t-test was used to compare two groups, and one-way ANOVA was used for comparisons among three or four groups, followed by Dunnett’s multiple comparisons tests. Kaplan–Meier estimates were performed with the log-rank test. Data are represented as mean ± SD. A p-value of less than 0.05 was considered statistically significant.
3. Results
3.1. Oral Administration of 5-ALA Did Not Prevent the Development of Diabetes in NOD Mice
To investigate the impact of oral administration of 5-ALA/SFC on the development of autoimmune diabetes in NOD mice, we assessed diabetes incidence in NOD mice in response to 5-ALA administration with varying therapeutic periods and dosages. In NOD mice, islet autoimmunity is initiated by the infiltration of innate immune cells, such as dendritic cells, into islets, followed by the recruitment of autoreactive T cells specific to islet autoantigens [31]. NOD mice develop insulitis from 3–4 weeks of age and progress to diabetes due to the progressive destruction of β cells by antigen-specific T cells from 12 weeks of age [32,33]. The therapeutic schedule was tailored based on the disease stage, considering the different immune cells involved at each stage.
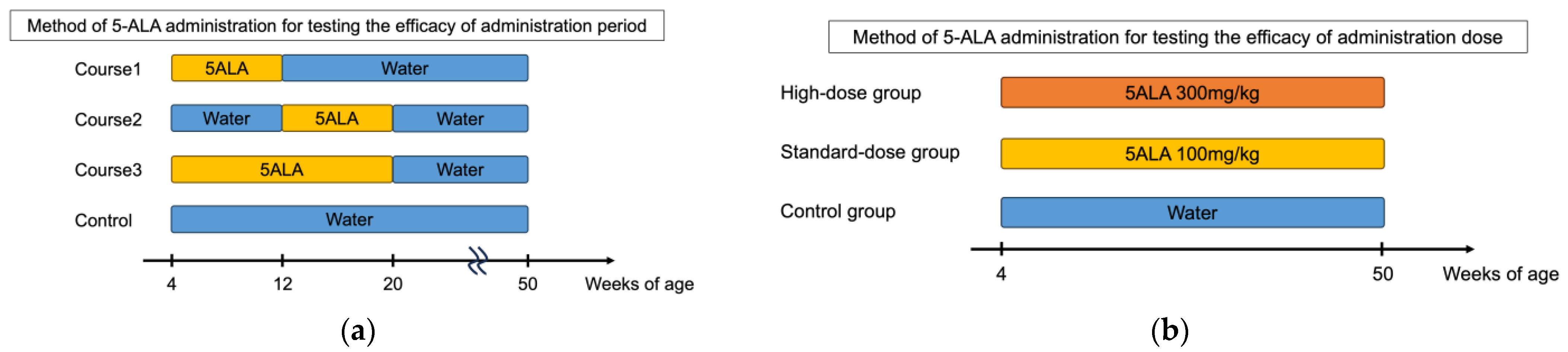
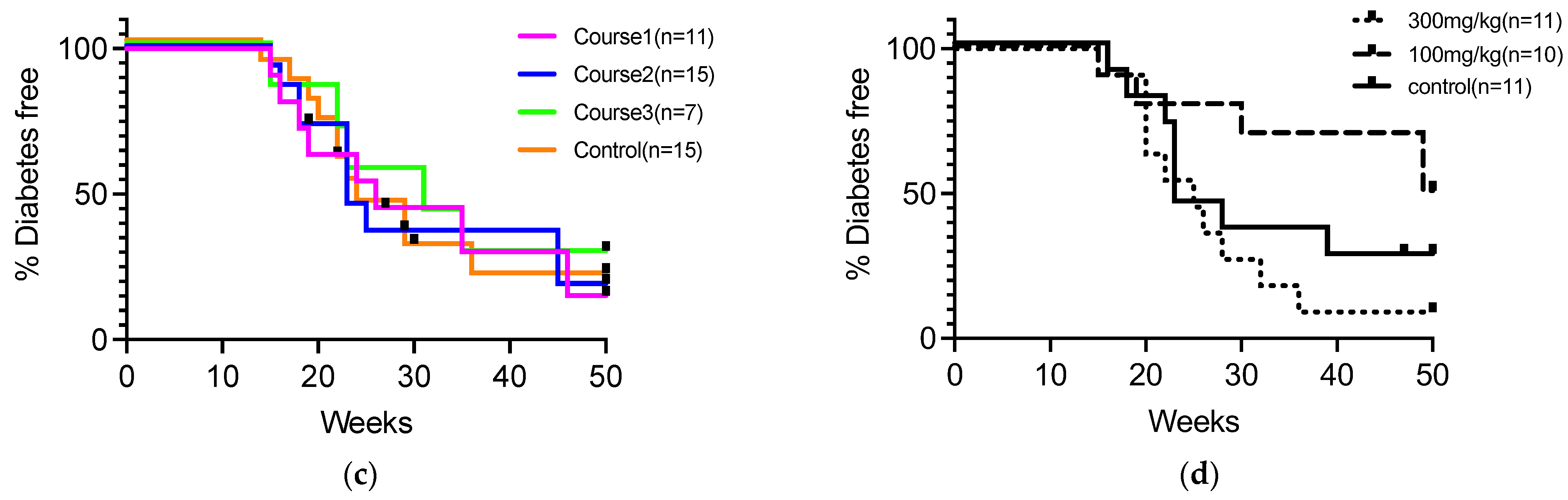
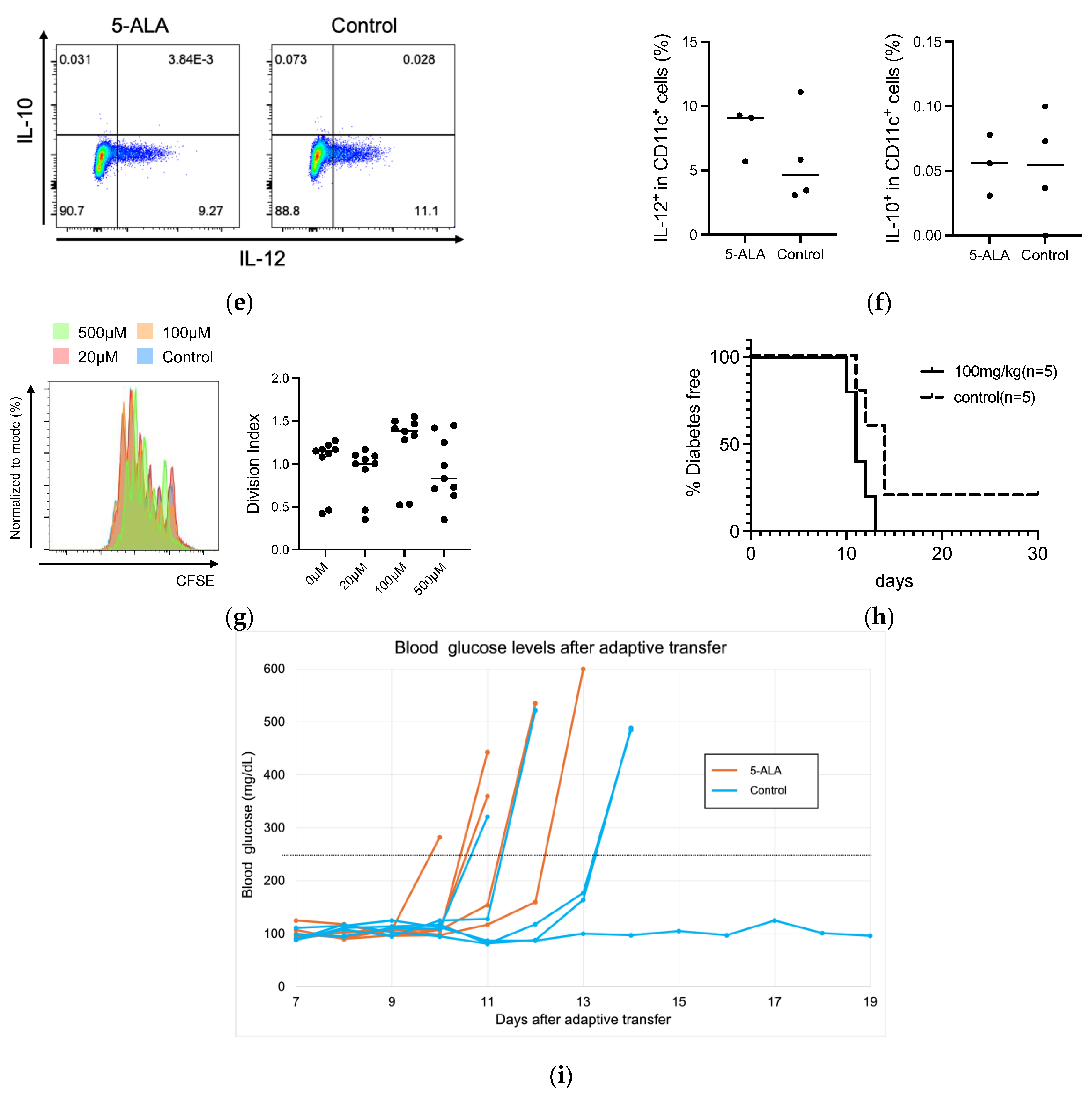
We assessed diabetes incidence in NOD mice in response to 5-ALA administration with varying therapeutic periods and dosages (Figure 1a,b). Initially, 5-ALA/SFC was administered temporarily to NOD mice at different stages of autoimmune diabetes, and the incidence of diabetes was monitored up to 50 weeks of age. The mice were given 100 mg/kg/day of 5-ALA/SFC from insulitis formation (4 weeks of age) to early diabetes (12 weeks of age) in Course 1 (n = 11), from early diabetes (12 weeks of age) to 20 weeks of age in Course 2 (n = 15), and from insulitis formation (4 weeks of age) to 20 weeks of age in Course 3 (n = 7). The incidence of diabetes did not significantly differ in each 5-ALA-treated group compared to the control group (Figure 1a). The incidence of diabetes did not significantly differ in each 5-ALA-treated group compared to the control group (Figure 1c). Next, 5-ALA/SFC was administered to NOD mice at two different doses. The NOD mice were divided into a standard-dose group (100 mg/kg, n = 11), a high-dose group (300 mg/kg, n = 10), and a control group (n = 11). The 5-ALA-treated group received oral administration of 5-ALA from the age of 4 weeks throughout the observation period. Diabetes incidence was monitored until 50 weeks of age. The diabetes incidence did not significantly differ in the high- and standard-dose groups compared to the control group (Figure 1d). We also examined blood glucose levels in the groups during the prediabetic period (Supplementary Figure S1). No statistically significant differences in blood glucose levels were observed among the groups at 4, 5, 8, 10, and 12 weeks of age.

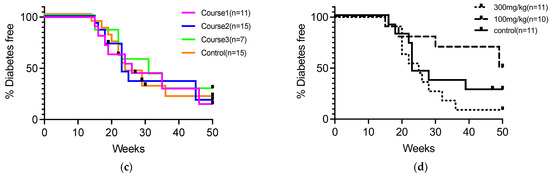
Figure 1.
Cumulative incidence of diabetes in nonobese diabetic (NOD) mice following variations in the duration and dosage of 5-aminolevulinic acid (5-ALA) administration. (a) Schematic representation of the 5-ALA administration schedule to assess the effect of treatment duration. NOD mice received 100 mg/kg/day of 5-ALA/sodium ferrous citrate (SFC) during three different periods: from 4 to 12 weeks of age (Course 1, n = 11), from 12 to 20 weeks of age (Course 2, n = 15), and from 4 to 20 weeks of age (Course 3, n = 7). The incidence of diabetes in each group was compared with that in untreated controls (n = 15). (b) Schematic representation of the 5-ALA administration schedule to evaluate its dose-dependent effects. NOD mice received different doses of 5-ALA/SFC from 4 to 50 weeks of age: the high-dose group (300 mg/kg, n = 11), the standard-dose group (100 mg/kg, n = 10), and the control group (n = 11). (c) Cumulative incidence of diabetes up to 50 weeks of age in mice treated with 5-ALA for different durations, as described in (a). Course 1 is indicated by pink lines, Course 2 by blue lines, Course 3 by green lines, and the control group by orange lines. (d) The cumulative incidence of diabetes up to 50 weeks of age in mice treated with different doses of 5-ALA, as described in (b). The high-dose group is represented by dotted lines, the standard-dose group by dashed lines, and the control group by solid lines. Statistical analysis of the Kaplan–Meier estimates using the log-rank test revealed no significant difference between the treatment and control groups in (c,d).
3.2. The IAA Production and Severity of Insulitis Were Not Suppressed by 5-ALA Administration in NOD Mice
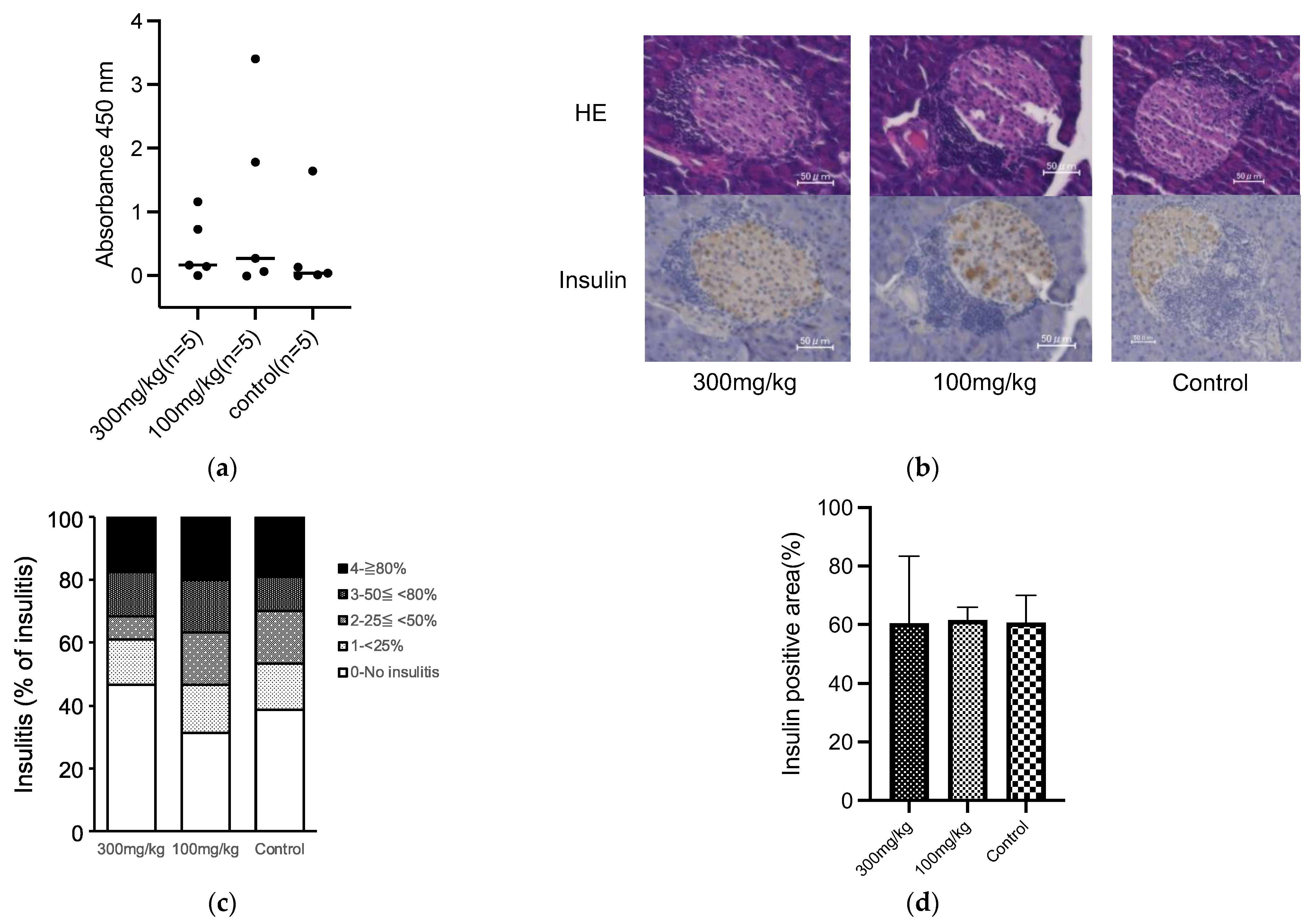
The impact of oral 5-ALA administration on insulitis and IAA development was assessed in NOD mice. NOD mice were given 5-ALA/SFC at standard (100 mg/kg) and high (300 mg/kg) doses from 4 weeks of age. NOD mice typically start to progress diabetes from 12 weeks of age in our animal facility, and it was reported that the level of IAA peaked at 8 to 12 weeks of age [34]. Therefore, we examined the level of insulitis and IAA at 12 weeks of age. The IAA production did not significantly differ in each group from that in the control group (0.44 ± 0.49 in the high-dose group, 0.48 ± 1.10 in the standard-dose group, 0.36 ± 0.72 in the control group) (Figure 2a). Both groups of 5-ALA-treated NOD mice showed similar levels of insulitis and residual β cell mass at 12 weeks of age compared to the control group (Residual β cell mass; 60.44 ± 20.94% in the high-dose group, 61.65 ± 4.32% in the standard-dose group, 60.72 ± 9.31% in the control group) (Figure 2b–d).
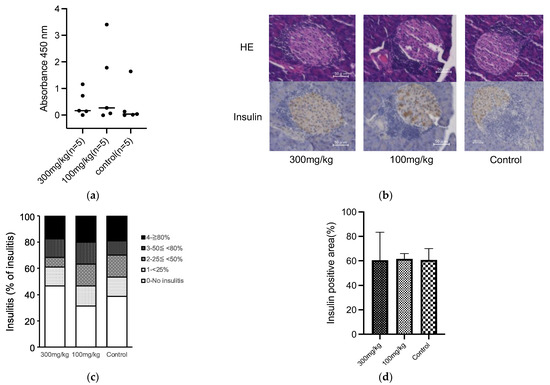
Figure 2.
(a) Serum insulin autoantibody (IAA) levels represented as absorbance at 450 nm in the 5-aminolevulinic acid (5-ALA)/sodium ferrous citrate (SFC) high-dose (300 mg/kg), standard-dose (100 mg/kg), and control groups at 12 weeks of age (n = 5 per group). Black horizontal lines indicate mean values. (b) Representative sections of pancreatic islets from nonobese diabetic (NOD) mice at 12 weeks of age in each of the 5-ALA/SFC and control groups. Pancreatic sections were assessed by hematoxylin and eosin and IHC staining to determine insulin expression. Scale bars, 50 μm (magnification, 400×). (c) The severity of insulitis in the 5-ALA/SFC high-dose (300 mg/kg), standard-dose (100 mg/kg), and control groups at 12 weeks of age (n = 6 per group). Insulitis severity was scored as previously described [27]. Insulitis levels are represented by different colors: 0 (white), 1 (light gray), 2 (gray), 3 (dark gray), and 4 (black). (d) Quantification of insulin-positive area in each group at 12 weeks of age. Data are shown as mean ± SD (5-ALA/SFC 300 mg/kg group n = 4, 100 mg/kg group n = 4, and control group n = 5). One-way ANOVA revealed no statistical difference, and the Dunnett’s multiple comparisons test showed no significant difference between the treatment and control groups in (a,d).
3.3. HO-1 Expression and Effector Function in Immune Cells Remained Unchanged by Short-Term 5-ALA Administration
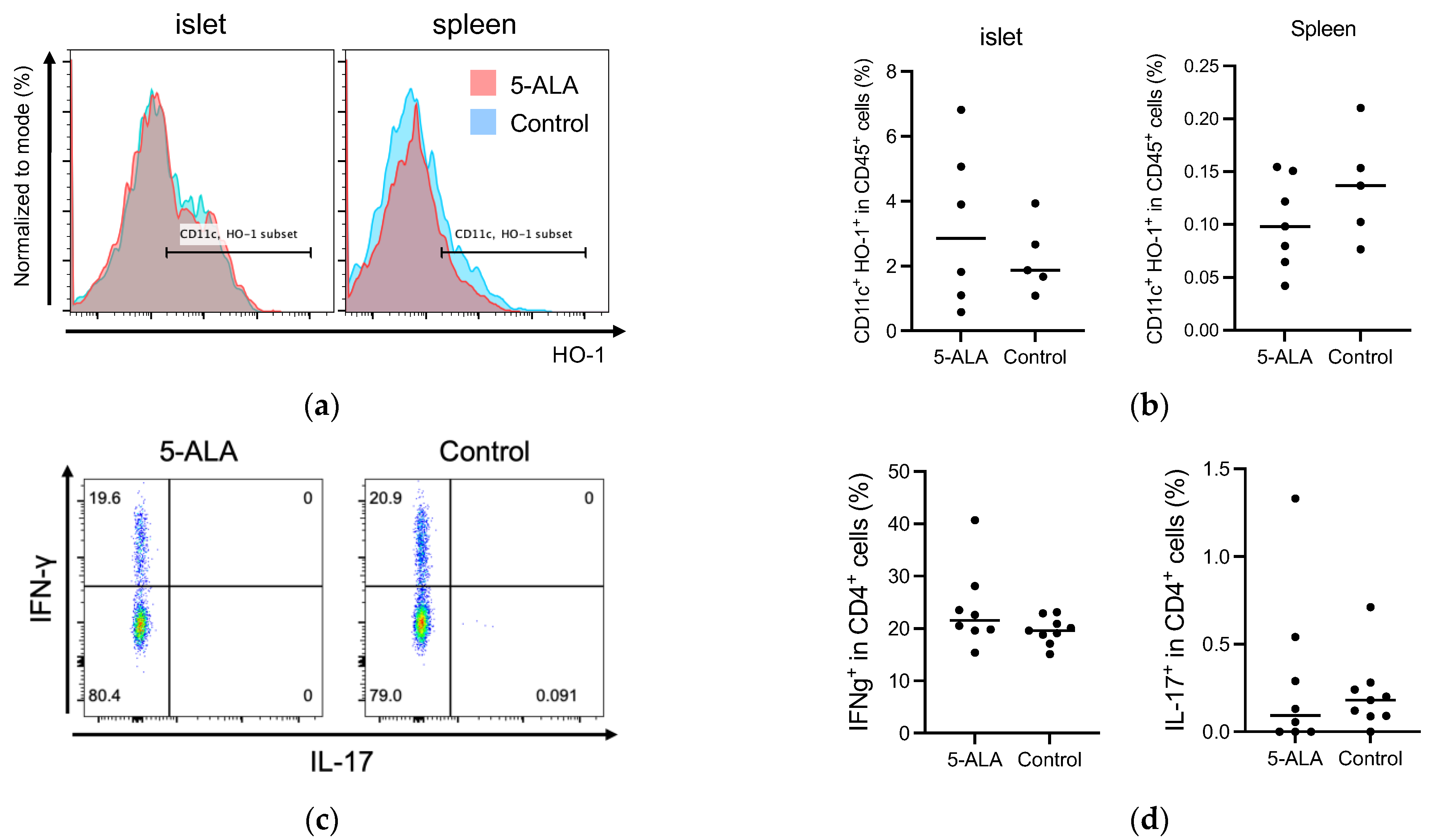
Our results suggested that oral administration of 5-ALA had a limited effect on the development of diabetes and insulitis, and IAA production in NOD mice (Figure 1 and Figure 2). Previous reports have documented that HO-1 inducers decrease the production of pro-inflammatory cytokines, IL-12 and IL-6, in dendritic cells [10,15]. These cells are potentially influential in determining the fate of T-cell subsets, such as IFN-γ-producing cells (Th1) and IL-17-producing cells (Th17). Subsequently, we evaluated whether oral 5-ALA administration affected HO-1 expression in dendritic cells in islets and spleens, cytokine production by CD4+ T cells originating from islets and by dendritic cells from the spleen, and cell proportion in immune cells responsible for the pathogenesis of autoimmune diabetes. Non-diabetic NOD mice over 12 weeks of age were divided into a control group and a treatment group that received 5-ALA. The 5-ALA-treated groups received oral administration of 5-ALA at a dosage of 100 mg/kg for 1 week prior to the experiments. No significant differences were observed in HO-1 expression in dendritic cells in the islets and spleen (Islet: 3.21 ± 2.46% in the treatment group, 2.24 ± 1.1% in the control group; Spleen: 0.1 ± 0.04% in the treatment group, 0.14 ± 0.05% in the control group) (Figure 3a,b); IFN-γ/IL-17 production by CD4+ T cells in the islets (IFN-γ: 23.78 ± 7.75% in the treatment group, 19.63 ± 2.56% in the control group; IL-17: 0.29 ± 0.46% in the treatment group, 0.21 ± 0.20% in the control group) (Figure 3c,d); IL-12/IL-10 production by dendritic cells isolated from splenocytes (IL-12: 8.02 ± 2.02% in the treatment group, 5.86 ± 3.7% in the control group; IL-10: 0.06 ± 0.02% in the treatment group, 0.05 ± 0.04% in the control group) (Figure 3e,f).
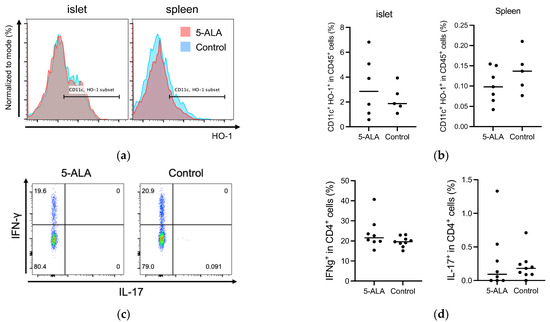
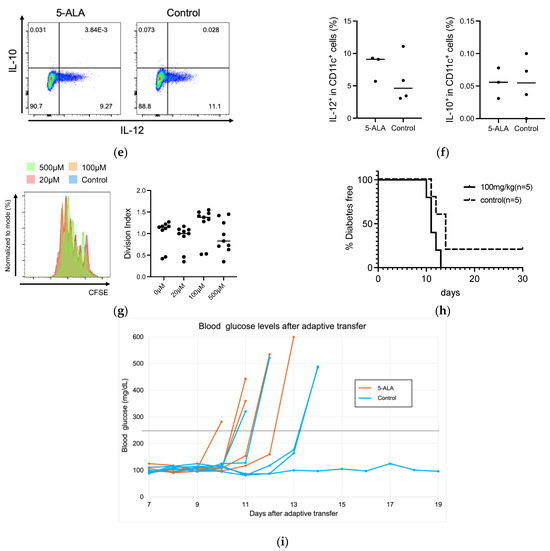
Figure 3.
(a,b) Flow cytometry analysis of heme oxygenase-1 (HO-1) expression in dendritic cells in the islets and spleen of 5-Aminolevulinic acid (5-ALA)-treated or untreated nonobese diabetic NOD mice. Representative plot (a) and quantification (b) of HO-1+ dendritic cells among CD45+ cells in the islet (5-ALA: n = 6; control: n = 5) and spleen (5-ALA: n = 7; control: n = 5) from 5-ALA-treated or untreated mice. (c,d) Representative plot (c) and quantification (d) of IFN-γ+ and IL-17+ cells among CD4+ cells in the islet (5-ALA: n = 8; control: n = 9). (e,f) Representative plot (e) and quantification (f) of IL-10+ or IL-12+ cells among CD11c+ cells in the spleen (5-ALA: n = 3; control: n = 4) (g). Division of CFSE-labeled BDC2.5 CD4+ T cells 4 days after stimulation with an islet antigen. Representative data and division index (average number of cell divisions) are shown (500 μM, 100 μM, 20 μM, or untreated; n = 9 per group) (h) The cumulative incidence of diabetes after the adoptive transfer of antigen-specific CD4+ T cells from BDC2.5-NOD mice into Rag1KO-NOD recipient mice (n = 5 per group; 5ALA: solid line, control: dashed line). (i) Line graph showing blood glucose levels corresponding to (h). The dotted line indicates a blood glucose level of 250 mg/dL. Values exceeding 600 mg/dL are plotted as 600 mg/dL. The blood glucose levels of 5-ALA-treated group are shown as orange solid lines, and the control group as blue solid lines. All the treated mice used for experiments in (a–f) received 1 week of oral administration of 5-ALA (100 mg/kg). The unpaired Student’s t-test revealed no significant difference between the treatment and control groups in (b,d,f). One-way ANOVA revealed no statistical difference, and the Dunnett’s multiple comparisons test showed no significant difference between the treatment and control groups in (g). Statistical analysis of the Kaplan–Meier estimates using the log-rank test revealed no significant difference between the treatment and control groups in (h).
Previous reports have also documented that HO-1 inducers suppress the expression of major histocompatibility complex (MHC) and co-stimulatory molecules in dendritic cells and reduce their capacity to stimulate T cells in vitro [10,11,15]. It has been reported that the capacity of an HO-1 inducer to suppress T-cell stimulation by dendritic cells in vitro is linked to the suppression of diabetes progression following the adoptive transfer of dendritic cells treated with the HO-1 inducer [35]. To evaluate the effect of 5-ALA on antigen stimulation in vitro, the proliferation of antigen-specific CD4+ T cells after antigen stimulation in the presence of 5-ALA was compared with that of the control group (Figure 3g). No significant difference was observed in the proliferation of BDC2.5 CD4+ T cells at any 5-ALA concentration (Division index: 1.00 ± 0.33 at 0 μM, 0.91 ± 0.29 at 20 μM, 1.22 ± 0.4 at 100 μM, 0.93 ± 0.38 at 500 μM). Finally, we evaluated the incidence of diabetes in Rag-1 KO NOD mice treated with 5-ALA for 4 weeks and transferred with naïve CD4+ T cells. No significant difference was observed in the cumulative incidence of diabetes in the recipients (Rag-1 KO NOD mice) with or without 5-ALA treatment (Figure 3h,i).
4. Discussion
In this study, oral administration of 5-ALA/SFC did not suppress the development of autoimmune diabetes in NOD mice. It did not reduce insulitis levels, insulin autoantibody production, or diabetes induction by diabetogenic BDC2.5 CD4+ T cells.
The histological evaluation revealed no difference in insulitis levels, indicating that dendritic cell migration to the islet region, antigen presentation to T cells, and T-cell recruitment were not affected by 5-ALA treatment. Similarly, no differences were observed in IAA levels, suggesting that the development of insulin-specific B cells was not suppressed. These findings suggest that the suppressive effect of oral 5-ALA/SFC on dendritic cell-mediated islet autoimmunity is limited for T and B cells in the pathogenesis of autoimmune diabetes in NOD mice. Our results of similar HO-1 expression and cytokine production in T and dendritic cells in 5-ALA-treated mice support this interpretation (Figure 3). In a different autoimmune disease model of adult-onset Still’s disease, oral administration of 5-ALA/SFC was found to induce anti-inflammatory properties in monocytes and M2 macrophages, ameliorating disease activity in collagen-induced arthritis and macrophage activation syndrome [25]. Notably, the mechanism of HO-1 induction by 5-ALA/SFC in macrophages has been well-studied [8]. The varying efficacy of immune modulation in autoimmune diseases may be due to the specific immune cell types involved in disease progression, as macrophages are not crucial for inducing autoimmune diabetes in NOD mice.
In the adoptive transfer experiment, the diabetes-inducing capacity of islet antigen-specific CD4+ T cells was not suppressed by the administration of 5-ALA/SFC when transferred into immunodeficient (Rag1KO-NOD) mice, suggesting that antigen presentation by immune cells in the recipients and the acquisition of effector function of islet antigen-specific naïve CD4+ T cells were not suppressed. Hypothetically, 5-ALA treatment could induce a tolerogenic phenotype in dendritic cells and suppress the effector differentiation and proliferation of T cells; however, we found no significant changes in HO-1 expression in dendritic cells, cytokine production in dendritic and T cells, and proliferative activity in BDC2.5 CD4+ T cells in vitro. These findings indicate that oral 5-ALA administration may be insufficient for inducing tolerogenic dendritic cells in NOD mice.
A previous study demonstrated that simultaneous intradermal administration of autoantigens and a HO-1 inducer (CoPP) suppressed autoimmune diabetes, where autoreactive OT-1 T cells directly attacked beta cells expressing membrane-bound ovalbumin (OVA) under the control of the insulin promoter in RIP-OVAhigh mice [22]. Similarly, in the report of tolerogenic dendritic cells genetically modified with lentiviral vectors co-encoding antigen-derived peptides and interleukin 10, the expression of autoantigens as well as immunosuppressive molecules in dendritic cells might be necessary to promote antigen-specific tolerance for islets [36]. Therefore, our study, in which 5-ALA was administered orally without autoantigens, may have had limited efficacy in inducing tolerogenic dendritic cells and suppressing pancreatic islet autoimmunity.
Recently, remarkable progress has been made in the field of immunotherapy for patients with T1D [4,37,38]. However, the decline in beta cell function in patients with T1D is not completely halted. Therefore, there is a need to develop adjunctive therapies with higher safety to maintain long-term efficacy. Although oral administration of 5-ALA had limited effects in this study, several HO-1 inducers, including 5-ALA, have emerged as candidates for better-tolerated immunomodulatory therapy for autoimmune diseases due to their low toxicity.
The limitations of this study are that 5-ALA/SFC was administered voluntarily through water supply bottles, so there is a possibility that the actual dosage of 5-ALA/SFC may have varied. In addition, due to ad libitum administration, 5-ALA/SFC was administered after weaning, starting at 4 weeks of age. HO-1 and cytokine expressions in immune cells were evaluated after 1 week of 5-ALA/SFC administration.
In conclusion, the impact of oral administration of 5-ALA/SFC on the development of autoimmune diabetes and autoimmunity in pancreatic islets in NOD mice is limited, and further analysis is required.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diabetology6070062/s1. Figure S1. Blood glucose levels in NOD mice from 4 to 12 weeks of age in the 5-ALA-treated and control groups.
Author Contributions
Conceptualization: A.K. and N.A.; methodology: S.N., S.A., T.N., M.K., S.-I.I., and K.M.; formal analysis and investigation: S.N., T.N., S.-I.I., K.M., A.H., and I.H.; writing—original draft preparation: S.N.; writing—review and editing: S.A.; funding acquisition and resources: A.K. and N.A.; supervision: T.A., M.N., M.O., A.K., and N.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by KIYAN PHARMA Co., Ltd., Tokyo, Japan. The 5-ALA/SFC was provided by KIYAN PHARMA Co., Ltd., Tokyo, Japan.
Institutional Review Board Statement
All animal experiments were approved by the Ethics Review Committee for Animal Experimentation (Institutional Animal Care and Use Committee [IACUC]), Nagasaki University, under protocol number: # 2411011983 (Approval Date: 1 November 2024).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be available upon request.
Acknowledgments
We would like to thank M. Masaki (Nagasaki University, Nagasaki, Japan) for the technical assistance with genotyping and animal husbandry. We would also like to thank Kiyoshi Kita at Nagasaki University School of Tropical Medicine and Global Health for his helpful suggestions regarding this study.
Conflicts of Interest
This work was partially supported by KIYAN PHARMA Co., Ltd., Tokyo, Japan. The 5-ALA/SFC was provided by KIYAN PHARMA Co., Ltd., Tokyo, Japan. Shinpei Nishikido, Satoru Akazawa, Tetsuro Niri, Atsushi Kawakami, and Norio Abiru declare that this study received funding from KIYAN PHARMA Co., Ltd., Tokyo, Japan. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Abbreviations
The following abbreviations are used in this manuscript:
| 5-ALA | 5-Aminolevulinic Acid |
| CFSE | Carboxyfluorescein succinimidyl ester |
| CoPP | Cobalt protoporphyrin IX |
| HO-1 | Home oxygenase-1 |
| IAA | Insulin autoantibody |
| IHC | Immunohistochemistry |
| NOD | Nonobese diabetic |
| SFC | Sodium ferrous citrate |
| T1D | Type 1 diabetes |
References
- Bluestone, J.A.; Herold, K.; Eisenbarth, G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature 2010, 464, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Bluestone, J.A.; Buckner, J.H.; Herold, K.C. Immunotherapy: Building a bridge to a cure for type 1 diabetes. Science 2021, 373, 510–516. [Google Scholar] [CrossRef]
- Herold, K.C.; Bundy, B.N.; Long, S.A.; Bluestone, J.A.; DiMeglio, L.A.; Dufort, M.J.; Gitelman, S.E.; Gottlieb, P.A.; Krischer, J.P.; Linsley, P.S.; et al. An Anti-CD3 Antibody, Teplizumab, in Relatives at Risk for Type 1 Diabetes. N. Engl. J. Med. 2019, 381, 603–613. [Google Scholar] [CrossRef]
- Ramos, E.L.; Dayan, C.M.; Chatenoud, L.; Sumnik, Z.; Simmons, K.M.; Szypowska, A.; Gitelman, S.E.; Knecht, L.A.; Niemoeller, E.; Tian, W.; et al. Teplizumab and beta-Cell Function in Newly Diagnosed Type 1 Diabetes. N. Engl. J. Med. 2023, 389, 2151–2161. [Google Scholar] [CrossRef] [PubMed]
- Rehani, P.R.; Iftikhar, H.; Nakajima, M.; Tanaka, T.; Jabbar, Z.; Rehani, R.N. Safety and Mode of Action of Diabetes Medications in comparison with 5-Aminolevulinic Acid (5-ALA). J. Diabetes Res. 2019, 2019, 4267357. [Google Scholar] [CrossRef] [PubMed]
- Higashikawa, F.; Noda, M.; Awaya, T.; Tanaka, T.; Sugiyama, M. 5-aminolevulinic acid, a precursor of heme, reduces both fasting and postprandial glucose levels in mildly hyperglycemic subjects. Nutrition 2013, 29, 1030–1036. [Google Scholar] [CrossRef]
- Fujino, M.; Nishio, Y.; Ito, H.; Tanaka, T.; Li, X.K. 5-Aminolevulinic acid regulates the inflammatory response and alloimmune reaction. Int. Immunopharmacol. 2016, 37, 71–78. [Google Scholar] [CrossRef]
- Nishio, Y.; Fujino, M.; Zhao, M.; Ishii, T.; Ishizuka, M.; Ito, H.; Takahashi, K.; Abe, F.; Nakajima, M.; Tanaka, T.; et al. 5-Aminolevulinic acid combined with ferrous iron enhances the expression of heme oxygenase-1. Int. Immunopharmacol. 2014, 19, 300–307. [Google Scholar] [CrossRef]
- Moreau, A.; Hill, M.; Thebault, P.; Deschamps, J.Y.; Chiffoleau, E.; Chauveau, C.; Moullier, P.; Anegon, I.; Alliot-Licht, B.; Cuturi, M.C. Tolerogenic dendritic cells actively inhibit T cells through heme oxygenase-1 in rodents and in nonhuman primates. FASEB J. 2009, 23, 3070–3077. [Google Scholar] [CrossRef]
- Chauveau, C.; Remy, S.; Royer, P.J.; Hill, M.; Tanguy-Royer, S.; Hubert, F.X.; Tesson, L.; Brion, R.; Beriou, G.; Gregoire, M.; et al. Heme oxygenase-1 expression inhibits dendritic cell maturation and proinflammatory function but conserves IL-10 expression. Blood 2005, 106, 1694–1702. [Google Scholar] [CrossRef]
- Listopad, J.; Asadullah, K.; Sievers, C.; Ritter, T.; Meisel, C.; Sabat, R.; Docke, W.D. Heme oxygenase-1 inhibits T cell-dependent skin inflammation and differentiation and function of antigen-presenting cells. Exp. Dermatol. 2007, 16, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, A.; Wafula, P.O.; Teles, A.; El-Mousleh, T.; Linzke, N.; Zenclussen, M.L.; Langwisch, S.; Heinze, K.; Wollenberg, I.; Casalis, P.A.; et al. Blockage of heme oxygenase-1 abrogates the protective effect of regulatory T cells on murine pregnancy and promotes the maturation of dendritic cells. PLoS ONE 2012, 7, e42301. [Google Scholar] [CrossRef]
- Al-Huseini, L.M.; Aw Yeang, H.X.; Hamdam, J.M.; Sethu, S.; Alhumeed, N.; Wong, W.; Sathish, J.G. Heme oxygenase-1 regulates dendritic cell function through modulation of p38 MAPK-CREB/ATF1 signaling. J. Biol. Chem. 2014, 289, 16442–16451. [Google Scholar] [CrossRef] [PubMed]
- George, J.F.; Braun, A.; Brusko, T.M.; Joseph, R.; Bolisetty, S.; Wasserfall, C.H.; Atkinson, M.A.; Agarwal, A.; Kapturczak, M.H. Suppression by CD4+CD25+ regulatory T cells is dependent on expression of heme oxygenase-1 in antigen-presenting cells. Am. J. Pathol. 2008, 173, 154–160. [Google Scholar] [CrossRef]
- Campbell, N.K.; Fitzgerald, H.K.; Malara, A.; Hambly, R.; Sweeney, C.M.; Kirby, B.; Fletcher, J.M.; Dunne, A. Naturally derived Heme-Oxygenase 1 inducers attenuate inflammatory responses in human dendritic cells and T cells: Relevance for psoriasis treatment. Sci. Rep. 2018, 8, 10287. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, X.; Xu, M.; Wu, X.; Zhao, F.; Zhao, C. Quercetin attenuates collagen-induced arthritis by restoration of Th17/Treg balance and activation of Heme Oxygenase 1-mediated anti-inflammatory effect. Int. Immunopharmacol. 2018, 54, 153–162. [Google Scholar] [CrossRef]
- Geisel, J.; Bruck, J.; Glocova, I.; Dengler, K.; Sinnberg, T.; Rothfuss, O.; Walter, M.; Schulze-Osthoff, K.; Rocken, M.; Ghoreschi, K. Sulforaphane protects from T cell-mediated autoimmune disease by inhibition of IL-23 and IL-12 in dendritic cells. J. Immunol. 2014, 192, 3530–3539. [Google Scholar] [CrossRef] [PubMed]
- Ghoreschi, K.; Bruck, J.; Kellerer, C.; Deng, C.; Peng, H.; Rothfuss, O.; Hussain, R.Z.; Gocke, A.R.; Respa, A.; Glocova, I.; et al. Fumarates improve psoriasis and multiple sclerosis by inducing type II dendritic cells. J. Exp. Med. 2011, 208, 2291–2303. [Google Scholar] [CrossRef]
- Yan, S.C.; Wang, Y.J.; Li, Y.J.; Cai, W.Y.; Weng, X.G.; Li, Q.; Chen, Y.; Yang, Q.; Zhu, X.X. Dihydroartemisinin Regulates the Th/Treg Balance by Inducing Activated CD4+ T cell Apoptosis via Heme Oxygenase-1 Induction in Mouse Models of Inflammatory Bowel Disease. Molecules 2019, 24, 2475. [Google Scholar] [CrossRef]
- Hu, C.M.; Lin, H.H.; Chiang, M.T.; Chang, P.F.; Chau, L.Y. Systemic expression of heme oxygenase-1 ameliorates type 1 diabetes in NOD mice. Diabetes 2007, 56, 1240–1247. [Google Scholar] [CrossRef]
- Li, M.; Peterson, S.; Husney, D.; Inaba, M.; Guo, K.; Kappas, A.; Ikehara, S.; Abraham, N.G. Long-lasting expression of HO-1 delays progression of type I diabetes in NOD mice. Cell Cycle 2007, 6, 567–571. [Google Scholar] [CrossRef]
- Simon, T.; Pogu, J.; Remy, S.; Brau, F.; Pogu, S.; Maquigneau, M.; Fonteneau, J.F.; Poirier, N.; Vanhove, B.; Blancho, G.; et al. Inhibition of effector antigen-specific T cells by intradermal administration of heme oxygenase-1 inducers. J. Autoimmun. 2017, 81, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, S.; Okano, S.; Nohara, H.; Nakano, H.; Shirasawa, N.; Naito, A.; Yamamoto, M.; Kelly, V.P.; Takahashi, K.; Tanaka, T.; et al. 5-aminolevulinic acid (ALA) deficiency causes impaired glucose tolerance and insulin resistance coincident with an attenuation of mitochondrial function in aged mice. PLoS ONE 2018, 13, e0189593. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yang, X.; Zhu, P.; Fujino, M.; Ito, H.; Takahashi, K.; Nakajima, M.; Tanaka, T.; Wang, J.; Zhuang, J.; et al. Combination of 5-aminolevulinic acid and iron prevents skin fibrosis in murine sclerodermatous graft-versus-host disease. Exp. Dermatol. 2018, 27, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Koga, T.; Sumiyoshi, R.; Tsuji, Y.; Kodama, K.; Endo, Y.; Furukawa, K.; Kawakami, A. Efficacy and safety of 5-aminolevulinic acid in adult-onset Still’s disease: A preclinical study in mice and a pilot study in humans. Clin. Immunol. 2023, 257, 109846. [Google Scholar] [CrossRef]
- Jhala, G.; Chee, J.; Trivedi, P.M.; Selck, C.; Gurzov, E.N.; Graham, K.L.; Thomas, H.E.; Kay, T.W.; Krishnamurthy, B. Perinatal tolerance to proinsulin is sufficient to prevent autoimmune diabetes. JCI Insight 2016, 1, e86065. [Google Scholar] [CrossRef]
- Akazawa, S.; Kobayashi, M.; Kuriya, G.; Horie, I.; Yu, L.; Yamasaki, H.; Okita, M.; Nagayama, Y.; Matsuyama, T.; Akbari, M.; et al. Haploinsufficiency of interferon regulatory factor 4 strongly protects against autoimmune diabetes in NOD mice. Diabetologia 2015, 58, 2606–2614. [Google Scholar] [CrossRef]
- Graham, K.L.; Fynch, S.; Papas, E.G.; Tan, C.; Kay, T.W.H.; Thomas, H.E. Isolation and Culture of the Islets of Langerhans from Mouse Pancreas. Bio-Protoc. 2016, 6, e1840. [Google Scholar] [CrossRef]
- Niri, T.; Inoue, S.I.; Akazawa, S.; Nishikido, S.; Miwa, M.; Kobayashi, M.; Yui, K.; Okita, M.; Kawakami, A.; Abiru, N. Essential role of interferon-regulatory factor 4 in regulating diabetogenic CD4+ T and innate immune cells in autoimmune diabetes in NOD mice. Clin. Exp. Immunol. 2024, 219, uxae093. [Google Scholar] [CrossRef]
- Jin, N.; Wang, Y.; Crawford, F.; White, J.; Marrack, P.; Dai, S.; Kappler, J.W. N-terminal additions to the WE14 peptide of chromogranin A create strong autoantigen agonists in type 1 diabetes. Proc. Natl. Acad. Sci. USA 2015, 112, 13318–13323. [Google Scholar] [CrossRef]
- Pearson, J.A.; Wong, F.S.; Wen, L. The importance of the Non Obese Diabetic (NOD) mouse model in autoimmune diabetes. J. Autoimmun. 2016, 66, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Andre, I.; Gonzalez, A.; Wang, B.; Katz, J.; Benoist, C.; Mathis, D. Checkpoints in the progression of autoimmune disease: Lessons from diabetes models. Proc. Natl. Acad. Sci. USA 1996, 93, 2260–2263. [Google Scholar] [CrossRef] [PubMed]
- Delovitch, T.L.; Singh, B. The nonobese diabetic mouse as a model of autoimmune diabetes: Immune dysregulation gets the NOD. Immunity 1997, 7, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Abiru, N.; Yu, L.; Miao, D.; Maniatis, A.K.; Liu, E.; Moriyama, H.; Eisenbarth, G.S. Transient insulin autoantibody expression independent of development of diabetes: Comparison of NOD and NOR strains. J. Autoimmun. 2001, 17, 1–6. [Google Scholar] [CrossRef]
- Yang, X.; Ma, Z.; Tan, X.; Shi, Y.; Yuan, M.; Chen, G.; Luo, X.; Hou, L. Adoptive transfer of immature dendritic cells with high HO-1 expression delays the onset of T1DM in NOD mice. Life Sci. 2023, 335, 122273. [Google Scholar] [CrossRef]
- Passeri, L.; Andolfi, G.; Bassi, V.; Russo, F.; Giacomini, G.; Laudisa, C.; Marrocco, I.; Cesana, L.; Di Stefano, M.; Fanti, L.; et al. Tolerogenic IL-10-engineered dendritic cell-based therapy to restore antigen-specific tolerance in T cell mediated diseases. J. Autoimmun. 2023, 138, 103051. [Google Scholar] [CrossRef]
- Waibel, M.; Wentworth, J.M.; So, M.; Couper, J.J.; Cameron, F.J.; MacIsaac, R.J.; Atlas, G.; Gorelik, A.; Litwak, S.; Sanz-Villanueva, L.; et al. Baricitinib and beta-Cell Function in Patients with New-Onset Type 1 Diabetes. N. Engl. J. Med. 2023, 389, 2140–2150. [Google Scholar] [CrossRef]
- Pescovitz, M.D.; Greenbaum, C.J.; Krause-Steinrauf, H.; Becker, D.J.; Gitelman, S.E.; Goland, R.; Gottlieb, P.A.; Marks, J.B.; McGee, P.F.; Moran, A.M.; et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N. Engl. J. Med. 2009, 361, 2143–2152. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).