Abstract
Background: Insulin resistance (IR) is a metabolic disorder linked to type 2 diabetes and cardiovascular diseases. Visceral fat is a better predictor of IR than BMI and waist circumference due to its metabolic and inflammatory impact. Methods such as DEXA and bioimpedance (BIA) estimate body fat, while scales such as METS-IR, SPISE, and TyG assess IR risk. This study analyzes the utility of visceral and body fat measured by BIA compared to other indicators. Methods: A cross-sectional study was conducted on 8590 workers in the Balearic Islands, analyzing anthropometric, clinical, and analytical variables. Body fat and visceral fat were measured by bioimpedance, and insulin resistance was assessed using METS-IR, SPISE, and TyG. ROC curves were used to evaluate the predictive value of BMI, WC, and body fat. Results: The areas under the curve (AUCs) were highest for high METS-IR, particularly in women (>0.97), indicating excellent performance. TyG showed the lowest AUC, especially in men. Body and visceral fat showed the highest AUC for all IR scales. Youden’s indices were highest for high METS-IR, with good predictive capacity, while TyG showed low values, limiting its utility in predicting insulin resistance. Conclusions: Measuring body and visceral fat by BIA is superior to BMI or WC for estimating IR risk.
1. Introduction
Insulin resistance (IR) is a metabolic condition characterized by a reduced ability of cells to respond to insulin. This phenomenon is associated with an increased risk of developing various chronic diseases, including type 2 diabetes, cardiovascular diseases, and metabolic syndrome [1,2].
Several studies have demonstrated a strong correlation between a high body fat percentage and IR [3].
Although the mechanisms through which obesity induces insulin resistance are not yet fully understood, several mechanisms have been proposed, such as chronic inflammation of adipose tissue, mitochondrial dysfunction, and fat accumulation in the liver and muscle [4]. Several studies indicate that natural killer (NK) cells play a key role in obesity-induced inflammation and insulin resistance, being activated in epididymal white adipose tissue (eWAT). There, they promote the recruitment of macrophages, exacerbating inflammation and being associated with a higher risk of insulin resistance [5].
IR occurs in the early stages of obesity, regardless of the obesity phenotype, whether it be metabolically healthy obesity (MHO) or metabolically unhealthy obesity (MUO) [6].
Various anthropometric and body composition markers have been used as indirect indicators of IR, such as body mass index (BMI), waist circumference, total body fat, and visceral fat. Each of these metrics provides valuable information about the distribution and amount of fat in the body, aspects closely related to metabolic health [7].
Total body fat includes all the fat present in the body, including subcutaneous fat (beneath the skin) and visceral fat (surrounding internal organs). Although both contribute to energy storage and body protection, visceral fat has a greater impact on metabolic health [8]. Its proximity to vital organs and its ability to release free fatty acids directly into the liver link it to an increased risk of developing metabolic diseases such as type 2 diabetes and cardiovascular diseases [9].
Visceral fat is a more reliable predictor of IR compared to total body fat [10]. This difference is explained by the fact that visceral fat is more closely linked to inflammatory and metabolic processes that play a key role in the development of IR. According to a study by Després et al., the amount of visceral fat was shown to be a more accurate indicator of insulin sensitivity than BMI and waist circumference. These findings underscore the importance of specifically evaluating visceral fat in assessing the risk of developing IR [11].
BMI, widely used to classify nutritional status, correlates with IR, as excess adipose tissue, particularly in obesity (BMI ≥ 30 kg/m2) [12], increases lipotoxicity [13,14] and inflammatory stress [15,16], underlying mechanisms of insulin dysfunction. However, BMI has significant limitations [17]: it does not distinguish between lean mass and fat, nor does it identify the distribution of adipose tissue, critical factors in determining IR.
Waist circumference (WC) is a better predictor of IR than BMI, as it reflects abdominal fat accumulation [18]. Visceral adiposity, estimated by waist circumference or more advanced measurements such as densitometry, is closely related to metabolic dysfunction due to its high pro-inflammatory activity and impact on insulin sensitivity [19,20].
Total body fat measurement can be conducted using specialized methods that allow precise evaluation. These include dual-energy X-ray absorptiometry (DEXA), which provides detailed images of body composition [21]; air displacement plethysmography [22], which calculates the body fat percentage based on body density; and bioelectrical impedance, which estimates body fat from the electrical resistance of tissues [23].
Recently, metabolic risk scores such as METS-IR (metabolic score for insulin resistance), SPISE (single-point insulin sensitivity estimator), and the TyG index (triglyceride–glucose index) have gained relevance for their ability to estimate IR more accurately and practically.
The standard method for measuring insulin sensitivity is the euglycemic–hyperinsulinemic clamp technique, which is complex, costly, invasive, and not easily accessible. As a result, alternative methods such as indirect markers (TyG, METS-IR, SPISE) have been developed. The TyG index stands out due to its normal distribution in the studied population, making statistical analysis easier. Its simplicity, accessibility, low cost, high sensitivity, and specificity make it a useful alternative marker for insulin resistance. The TyG index shows superiority over other indices due to its relationship with glucotoxicity and lipotoxicity, key mechanisms in insulin resistance (IR). Hypertriglyceridemia contributes to the accumulation of fatty acids in non-adipose tissues, generating ectopic lipids and lipotoxicity. Moreover, visceral fat, with increased lipolysis and secretion of inflammatory adipokines, promotes an inflammatory state that interferes with insulin signaling [24].
METS-IR combines measures of triglycerides, fasting glucose, and body weight [25], providing a practical and accurate estimation of IR [26]. Its sensitivity is especially useful in populations with abdominal obesity, where elevated triglyceride and glucose levels are early markers of metabolic dysfunction [27]. Studies have shown that METS-IR correlates significantly with visceral fat and waist circumference, making it a powerful tool to identify individuals at high risk of IR and metabolic diseases [28,29].
SPISE uses HDL cholesterol, triglycerides, and BMI to estimate insulin sensitivity [30]. Although its application is more frequent in individuals with mild or moderate obesity, recent studies have indicated that SPISE is particularly effective in distinguishing between normal sensitivity and insulin resistance in young populations without established diabetes [31,32].
The TyG index, derived from triglyceride and glucose levels, has shown a strong association with IR [33] and visceral fat [34]. It is simple to calculate and highly reproducible, making it ideal for population studies and clinical settings with limited resources [35].
The assessment of insulin resistance risk benefits from a multidimensional approach that incorporates several measures of body composition and fat distribution. Combining these metrics allows for a more precise and comprehensive evaluation of metabolic risk. A person may have a normal BMI but a high amount of visceral fat, which would place them at an elevated risk of IR. Similarly, the combined use of BMI, waist circumference, and visceral fat measurements can better identify individuals at risk and guide preventive and therapeutic interventions.
The aim of our study is to evaluate the utility of visceral and total body fat, determined by bioimpedance, compared to waist circumference and BMI in estimating a high risk of IR.
2. Materials and Methods
2.1. Participants
A cross-sectional and descriptive study was carried out involving a total of 8590 employed individuals residing in the Balearic Islands, Spain. The participants were selected from among those who underwent their compulsory annual occupational health assessments between January 2019 and December 2020, facilitated by our occupational health and risk prevention service. This service provides coverage to a wide range of companies operating across multiple sectors, including healthcare, education, hospitality, construction, retail, transportation, public administration, industry, and cleaning services.
Inclusion criteria:
- Individuals aged between 18 and 69 years;
- Voluntary agreement to participate in the study;
- Provision of informed consent for the use of their data in epidemiological research;
- Active employment in one of the participating companies, without temporary work disability at the time of data collection.
Exclusion criteria:
- Age below 18 or above 69 years;
- Lack of employment in any of the companies involved in the research;
- Declining to participate or withholding consent for the use of personal data in the study;
- Did not provide consent for the use of their data in epidemiological analyses;
- Lacked essential variables required for the computation of clinical or diagnostic indices.
- The selection process for study participants is illustrated in the corresponding flowchart (Figure 1).
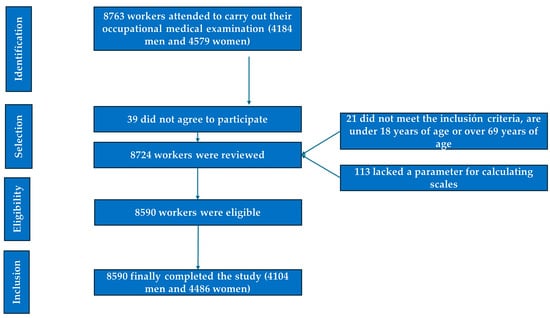 Figure 1. PRISMA diagram illustrating the participant selection process for this study.
Figure 1. PRISMA diagram illustrating the participant selection process for this study.
2.2. Variable Assessment
To minimize interobserver variability, all procedures were standardized in advance. Data collection, including anthropometric, clinical, and laboratory evaluations, was conducted by occupational health personnel affiliated with the participating companies.
- Sociodemographic and lifestyle data: age, sex, engagement in regular physical activity, weekly exercise frequency, and smoking habits.
- Anthropometric and clinical parameters: body weight, height, and waist and hip circumferences, as well as systolic and diastolic blood pressure.
- Laboratory markers: fasting plasma glucose, lipid profile, and liver enzyme levels.
2.2.1. Anthropometric Measurements
- Height (in centimeters) and body weight (in kilograms) were recorded using a SECA 700 mechanical scale and SECA 220 stadiometer, in accordance with International Society for the Advancement of Kinanthropometry (ISAK) guidelines [36].
- Waist circumference was assessed with the participant standing upright, with their feet together and abdomen relaxed. A SECA flexible measuring tape was used, positioned horizontally at the midpoint between the lowest palpable rib and the iliac crest. Hip circumference was measured at the point of greatest gluteal protrusion, also with the tape held parallel to the ground [37].
- Body composition, including total and visceral fat, was evaluated via bioelectrical impedance analysis (BIA), using a Tanita DC-430MA device. Elevated visceral fat was defined as values ≥10 on the bioimpedance scale, while thresholds for high total body fat were adjusted based on the participant’s age.
- The Tanita features an integrated auto-calibration system; however, certain steps can be followed to ensure that it functions optimally: Place the Tanita on a hard, flat, and level surface. If necessary, perform a reset. Periodic calibration should be conducted by technical service providers at least twice a year.
To ensure accurate measurements, validating the results is essential:
- User Preparation: Measurements should be performed under consistent conditions each time (e.g., at the same time of day, with the same hydration level, and preferably on an empty stomach or at least two hours after eating). The individual must be barefoot, must be wearing light clothing, and should not have engaged in intense physical activity beforehand.
- Comparison with Reference Standards: Repeated measurements should be performed on the same day to verify consistency. Additionally, body fat measurements should be compared with those obtained from a calibrated body composition device or validated reference methods (e.g., DEXA analysis or skinfold assessments performed by an expert).
- General Maintenance: The platform should be regularly cleaned with a soft, dry cloth. The use of harsh chemicals should be avoided. The device should be stored in a dry, stable environment to prevent damage to the sensors.
2.2.2. Clinical Determinations
- Blood Pressure Assessment: Arterial blood pressure was recorded using an OMRON M3 automated sphygmomanometer. Participants remained seated with their legs uncrossed and at rest for at least 10 min prior to measurement. Three consecutive readings were taken at one-minute intervals, and the mean value was used for analysis.
2.2.3. Biochemical Analyses
- Venous blood samples were drawn following a fasting period of no less than 12 h. The samples were processed as follows: “The tube used was the 8.5 mL BD SST II Vacutainer serum tube with separating gel, reference BD 366468. The samples were transported to the laboratory in a refrigerator (between 5 and 10 degrees Celsius). The samples were centrifuged in the laboratory within two hours of collection and immediately analyzed on an autoanalyzer” [38,39].
- LDL cholesterol concentrations were estimated using the Friedewald equation, applicable only when triglyceride levels were below 400 mg/dL. All biochemical variables are reported in milligrams per deciliter (mg/dL).
2.2.4. Insulin Resistance Risk Scales Applied
- Metabolic score for insulin resistance (METS-IR). METS-IR = Ln(2 × glucose) + triglycerides × BMI)/(Ln(HDL-c). High values are defined as 50 and above [40].
- SPISE = 600 × HDL0.185/triglycerides0.2 × BMI1.338. SPISE-IR = 10/SPISE. High risk is considered at 1.51 [41].
- The triglyceride–glucose (TyG) index is calculated using the following formula: Ln(triglycerides [mg/dL] × glucose [mg/dL]2). Values exceeding 8.81 are classified as high [42].
2.2.5. Sociodemographic Variables and Healthy Habits
Male and female were the two dichotomous variables making up gender.
The date of the medical examination was subtracted from the date of birth to determine age.
Individuals who had smoked at least one cigarette (or its equivalent in other forms) in the previous month, or who had quit smoking less than a year prior, were considered smokers [43].
Socioeconomic class was determined based on the recommendation of the Spanish Society of Epidemiology, following the 2011 National Classification of Occupations. Class I includes managers, directors, and university professionals; class II comprises intermediate vocations and self-employed individuals; and class III consists of manual workers [44].
The International Physical Activity Questionnaire (IPAQ) was employed to assess the level of physical activity. This self-administered survey evaluates the amount of exercise performed over the preceding seven days [45].
2.3. Statistical Analysis
Quantitative variables were summarized using means and standard deviations, with comparisons between groups conducted via Student’s t-test. Categorical variables were analyzed using the chi-square test to estimate prevalence rates. Receiver Operating Characteristic (ROC) curve analysis was used to establish the optimal thresholds for moderate and elevated cardiovascular age. This analysis included the calculation of the area under the curve (AUC), the determination of cut-off values based on the Youden index, and assessments of sensitivity and specificity. Pearson’s correlation coefficient was applied to evaluate linear associations between continuous variables, while Cohen’s kappa statistic was used to assess agreement between categorical scales. All statistical procedures were carried out using SPSS software, version 29.0. A p-value below 0.05 was considered indicative of statistical significance.
3. Results
Table 1 outlines the anthropometric and clinical characteristics of the study population. A total of 8590 workers were included in the analysis, comprising 4104 men (47.8%) and 4486 women (52.2%). The mean age of participants was slightly above 41 years, with the majority distributed between 30 and 49 years of age. Most individuals were classified within social class I. Approximately 15% of both male and female participants reported being active smokers. Regular engagement in physical activity was noted in 47.1% of men and 38.4% of women.

Table 1.
Sample characteristics.
Table 2 shows the mean values of body fat and visceral fat in both sexes. In both cases, we can observe that as the amount of fat increases, the risk of insulin resistance (IR) also increases, both for body fat and visceral fat. This increase is highly significant in all three formulas used (p < 0.0001). When evaluating individuals with very high body fat values and high visceral fat values measured by bioimpedance, the percentages are higher, in all three formulas used, in individuals with high values on the IR risk scales. The differences observed in all cases show high statistical significance (p < 0.001). However, we can also observe that a small proportion of the sample presents normal IR risk despite elevated body fat or visceral fat measured by bioimpedance.

Table 2.
Mean values and prevalence of very high body fat and high visceral fat according to RI scale values by sex.
In Figure 2 and Table 3, the areas under the curve (AUCs) are presented to evaluate the predictive value of body fat (blue line), visceral fat (green line), WC (violet line), and BMI (red line) in relation to the three criteria assessed for determining insulin resistance (IR) risk. It is observed that the AUCs are always higher for high METS-IR, both in men and in women, with a result exceeding 0.9 in all cases, indicating that the test result is very good. In women, the AUC is even higher than 0.97 across all four ROC curves, with an excellent test result, reaching 0.988 for body fat and 0.983 for visceral fat, approaching perfection. The lowest AUCs are obtained with the TyG formula, yet for women, they exceed 0.75 in all cases, which suggests a good result. In men, the AUCs are below 0.75 for BMI and WC, signaling a moderate test result.
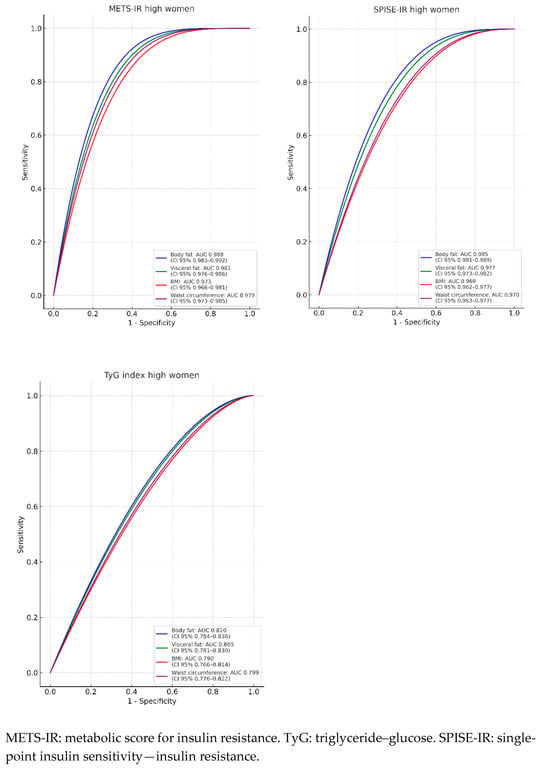
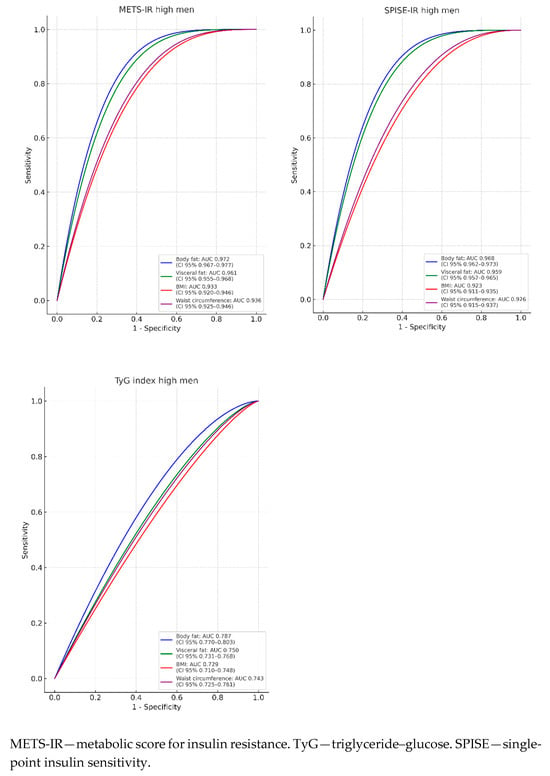
Figure 2.
ROC curves.

Table 3.
Areas under the curve for body fat, visceral fat, BMI, and WC to predict high values of RI scales by sex.
The highest AUCs are presented for body fat and visceral fat across the three IR risk scales evaluated.
The cut-off points for the four methods of assessing body fat are established in Table 4. The cut-off points for predicting high values of IR risk scales present the highest Youden indices for high METS-IR in both men and women. The Youden index ranges from 0.719 for BMI to 0.817 for body fat in men. Similar results are observed in women, with the Youden index ranging from 0.826 for BMI to 0.864 for body fat. These values indicate high predictive power in all cases and high reliability.

Table 4.
Cut-off points for body fat, visceral fat, BMI, and WC to predict high values of RI scales by sex.
On the other hand, the cut-off values for a high TyG index present a Youden index below 0.5 for all three formulas evaluated and for the four methods of assessing body fat. The highest Youden index for body fat is 0.492, and the lowest for BMI is 0.453, in women. In men, the Youden index for body fat is 0.441, and for BMI, it is 0.352, indicating that it is not useful for predicting IR or that it has limited applicability and should be used with caution.
The Pearson correlation coefficient shows moderate values between TyG, METS-IR, and SPISE-IR, while there is an excellent positive correlation between METS-IR and SPISE-IR (values are slightly higher in women). When studying Cohen’s kappa agreement index, it shows a low-to-moderate degree of agreement between TyG, METS-IR, and SPISE-IR, whereas there is very good agreement between METS-IR and SPISE-IR in both men and women, with higher values in the latter (Table 5).

Table 5.
Pearson’s correlation coefficient and Cohen’s kappa coefficient.
4. Discussion
Insulin resistance (IR) is a central factor in metabolic diseases such as type 2 diabetes, metabolic syndrome, and cardiovascular diseases. Traditional anthropometric markers, such as BMI and waist circumference, have been used as indirect indicators of IR but have limitations as they fail to reflect fat distribution, particularly visceral fat, which is key in the pathophysiology of IR [46,47]. In this context, scales such as METS-IR, SPISE, and TyG have emerged as more precise and practical tools [48]. These scales integrate metabolic components directly related to IR, overcoming the limitations of traditional indicators that inadequately capture the impact of visceral fat distribution, a crucial factor in IR pathophysiology.
In our sample, we analyzed two groups: 4104 men and 4486 women with similar characteristics. The average age was 41 years, and approximately 16% of the sample was smokers in both sexes, aligning with data from the Spanish National Institute of Statistics (INE) in 2022, where the national percentage of smokers was 17% [49]. However, regarding regular physical activity, our sample exhibited slightly higher rates (47.1% in men and 38.4% in women) compared to the INE data for 2022 (39.7% in men and 35.7% in women) [50].
The average values of body fat and visceral fat, differentiated by sex, are an important reflection of how body composition is associated with IR. The results reveal a clear trend: as body fat and visceral fat increase, the risk of IR rises significantly, underscoring the importance of considering these measures in metabolic assessments. Conversely, the discovery of a small proportion of individuals with normal IR risk (metabolically healthy obesity) [51] but very high body fat or visceral fat levels raises important questions. This could be attributed to genetic variations affecting insulin sensitivity or differences in fat distribution (e.g., subcutaneous versus visceral). This subgroup might benefit from additional monitoring to avoid overlooking latent metabolic risk, as, despite presenting lower metabolic risk than other individuals with obesity, their risk remains higher than that of metabolically healthy lean individuals [52]. Furthermore, these findings emphasize the importance of incorporating these measurements into risk population screenings. For instance, in individuals with high visceral fat levels, early intervention in terms of diet, physical activity, or pharmacological therapies could significantly impact the prevention of type 2 diabetes and other related diseases [53,54]. At the population level, these findings highlight the need for sex-specific preventive strategies, given that fat accumulation and its metabolic impact can differ considerably between men and women.
The analysis of the ROC curves provides valuable information about the predictive value of various anthropometric indicators for identifying IR risk, evaluated using three different scales. The areas under the curve (AUCs) reveal the clear superiority of indicators related to body and visceral fat, particularly when using the high METS-IR criterion, with results consistently exceeding the 0.9 threshold in both sexes. These findings underscore the ability of these measures to effectively discriminate between individuals at high and low IR risk. In women, the AUC values approach perfection, especially for body fat (0.988) and visceral fat (0.983). This suggests that these indicators not only are excellent predictive tools but may also be more sensitive to metabolic differences in women, possibly due to specific characteristics in fat distribution and its relationship with glucose metabolism. In men, although the AUC values are also high, they show slight variability depending on the criteria, with some indicators like BMI and waist circumference (WC) yielding more modest results (below 0.75).
Differences in body fat distribution between men and women influence glucose metabolism and the risk of metabolic diseases. Understanding these contrasts is key to developing sex-specific health strategies. Hormonal, genetic, and physiological factors determine these patterns: women tend to accumulate fat in the hips and thighs (gynoid distribution), whereas men are more likely to store fat in the abdominal region, both subcutaneous and visceral. Visceral fat, which surrounds internal organs, is associated with higher metabolic and cardiovascular risks. The location of adipose tissue directly affects insulin sensitivity. Visceral fat releases fatty acids and pro-inflammatory adipokines that can promote insulin resistance and increase the risk of type 2 diabetes. In contrast, subcutaneous fat, particularly in the lower body, has a more benign or even protective metabolic profile. Women generally exhibit greater insulin sensitivity than men, facilitating better blood glucose regulation. This metabolic advantage is partly attributed to estrogens, which enhance insulin action and promote healthier fat distribution. Additionally, during exercise, women oxidize more lipids and fewer carbohydrates than men, potentially contributing to greater glucose utilization efficiency and metabolic balance maintenance. However, in postmenopausal women, increased visceral fat raises the risk of metabolic syndrome and cardiovascular diseases. Therefore, incorporating these differences into prevention and treatment strategies could enhance their effectiveness [55].
The established cut-off points reinforce the clinical utility of these measures, especially for high METS-IR, where the Youden indices are consistently elevated, reaching values of up to 0.864 in women and 0.817 in men for body fat. This index reflects an optimal balance between sensitivity and specificity, solidifying body fat as a robust marker for identifying at-risk individuals. In contrast, the cut-off values for a high TyG index present Youden indices below 0.5, limiting its practical utility. This is particularly notable in men, where the highest Youden index is obtained for body fat (0.441), significantly lower than other methods and criteria. These results call into question the generalized applicability of the TyG index in clinical practice and suggest that its use should be complemented with more reliable indicators, particularly in male populations.
The AUC and cut-off point analyses underscore the relevance of including precise measures of body and visceral fat in metabolic risk assessment. This aligns with the literature supporting the central role of visceral fat as a key determinant of IR [56] due to its proximity to abdominal organs and its contribution to systemic inflammation and metabolic dysfunction. On the other hand, total body fat, while less specific, remains a useful marker, particularly in combination with other indicators [57,58]. The low accuracy of BMI [59] and WC, especially in men, raises questions about their continued use as primary tools in metabolic risk assessment. Although these measures are easy to obtain and widely used, they lack the specificity necessary to capture individual differences in body composition and fat distribution [60]. This may explain their poorer performance compared to more advanced methods, such as bioimpedance, which allows for a more direct assessment of visceral and body fat [61].
The results also reveal notable differences between men and women in the predictive capacity of different indicators. In women, higher AUC values suggest that the methods used are particularly effective in identifying IR risk. This could be related to biological differences in fat composition and distribution, as well as hormonal patterns influencing energy metabolism [62]. In contrast, in men, variability in results highlights the need to develop or adapt methods that are more sensitive to the specific characteristics of this group.
The findings of correlations and concordances between the TyG, METS-IR, and SPISE-IR indices provide key insights into their interrelationship and potential clinical utility. Pearson’s correlation coefficient shows that although TyG has a moderate relationship with METS-IR and SPISE-IR, its ability to reflect deeper similarities in metabolic risk assessment appears limited. This contrasts with the excellent positive correlation observed between METS-IR and SPISE-IR, suggesting that both indices share more robust underlying characteristics related to IR.
Regarding Cohen’s kappa concordance index, the results reinforce this difference. The low-to-moderate level of agreement between TyG and the other two indices could be related to the different variables considered in each formula and their sensitivity to specific factors such as fat distribution or triglyceride and glucose levels. In contrast, the very good concordance between METS-IR and SPISE-IR indicates that these indices consistently evaluate metabolic risk, particularly in women, where higher values may reflect sex-specific sensitivity.
These findings emphasize the need to prioritize indices with higher concordance and correlation, such as METS-IR and SPISE-IR, in the design of clinical and research strategies.
While BMI and WC will remain practical tools in primary care settings, scales such as METS-IR, SPISE, and TyG are emerging as standards in advanced research on and the management of metabolic diseases. Combining these tools with advanced imaging technologies and molecular biomarkers could revolutionize early IR diagnosis, enabling more personalized and effective interventions. The validation and adaptation of these scales in diverse populations will be essential for their global application.
5. Strengths and Limitations
Strengths of this study include the large sample size (almost 8600 people) and the use of both objective obesity scales (body and visceral fat) and validated scales that are widely used in most studies (BMI and waist circumference).
One of the primary limitations of this study is its cross-sectional design, which precludes the ability to draw causal inferences; thus, only associations between variables can be reported. Additionally, insulin resistance was assessed using validated clinical risk scores rather than direct biochemical or physiological measurements, which may limit the precision of the estimates.
Furthermore, since the study population was exclusively composed of workers residing in the Balearic Islands, the findings may not be generalizable to other geographic or demographic contexts. Therefore, caution should be exercised when attempting to extrapolate these results to different populations.
It would be useful to explore the possibility of combining indicators to improve diagnostic accuracy. For example, integrating visceral fat with biochemical markers could provide a more comprehensive assessment of metabolic risk, which we did not explore in this study.
6. Conclusions
Although BMI and waist circumference are effective in assessing insulin resistance, body fat and visceral fat assessed by bioelectrical impedance are more effective, with an AUC of almost 1.
This study reinforces the importance of body fat and visceral fat indicators as superior tools for assessing insulin resistance (IR) risk. Total body fat, visceral fat, BMI, and WC are essential tools for evaluating IR. Each of these measures provides a unique perspective on body composition and fat distribution, critical factors in assessing metabolic risk. The integration of these metrics into clinical practice can significantly improve the identification of individuals at risk and support the development of more effective prevention and management strategies.
These findings have important implications for clinical practice and research, highlighting the need for tailored and evidence-based strategies to effectively address metabolic risk.
Author Contributions
Conceptualization: Á.A.L.-G., J.I.R.-M. and M.G.S.; Data collection and analysis: Á.A.L.-G., M.G.S. and E.M.-A.R.; Data curation: Á.A.L.-G. and H.P.; Methodology: J.I.R.-M. and Á.A.L.-G.; Validation: M.T.V.-H., H.P. and P.J.T.L.; Formal analysis: Á.A.L.-G. and M.T.V.-H.; Investigation: M.G.S., M.T.V.-H. and P.J.T.L.; Draft: M.G.S.; Revision: J.I.R.-M. and Á.A.L.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study strictly adhered to the 2013 Declaration of Helsinki and ethical guidelines for research. Participants’ confidentiality and anonymity were always ensured. The Balearic Islands Research Ethics Committee (CEI-IB) approved the study under IB 4383/20 (approval date: 23 November 2020). Participants’ data were coded, and only the lead investigator knew their identities. All researchers involved in this study complied with Organic Law 3/2018, enacted on 5 December 2018, which guarantees the protection of digital rights and personal data. This law permits and ensures that study participants can access, correct, cancel, and object to the use of their data at any time.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
This study’s data are stored in a database that complies with all security measures at ADEMA-Escuela Universitaria. The Data Protection Delegate is Ángel Arturo López González.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Kosmas, C.E.; Bousvarou, M.D.; Kostara, C.E.; Papakonstantinou, E.J.; Salamou, E.; Guzman, E. Insulin resistance and cardiovascular disease. J. Int. Med. Res. 2023, 51, 3000605231164548. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gołacki, J.; Matuszek, M.; Matyjaszek-Matuszek, B. Link between Insulin Resistance and Obesity—From Diagnosis to Treatment. Diagnostics 2022, 12, 1681. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, S.H.; Park, S.Y.; Choi, C.S. Insulin Resistance: From Mechanisms to Therapeutic Strategies. Diabetes Metab. J. 2022, 46, 15–37. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, J. The emerging era of multidisciplinary metabolism research. Mol. Cells 2024, 47, 100032. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nathalie, G.; Bonamichi, B.D.S.F.; Kim, J.; Jeong, J.; Kang, H.; Hartland, E.R.; Eveline, E.; Lee, J. NK cell-activating receptor NKp46 does not participate in the development of obesity-induced inflammation and insulin resistance. Mol. Cells 2024, 47, 100007. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoddy, K.K.; Axelrod, C.L.; Mey, J.T.; Hari, A.; Beyl, R.A.; Blair, J.B.; Dantas, W.S.; Kirwan, J.P. Insulin resistance persists despite a metabolically healthy obesity phenotype. Obesity 2022, 30, 39–44. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pasanta, D.; Htun, K.T.; Pan, J.; Tungjai, M.; Kaewjaeng, S.; Chancharunee, S.; Tima, S.; Kim, H.J.; Kæwkhao, J.; Kothan, S. Waist Circumference and BMI Are Strongly Correlated with MRI-Derived Fat Compartments in Young Adults. Life 2021, 11, 643. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Calcaterra, V.; Biganzoli, G.; Ferraro, S.; Verduci, E.; Rossi, V.; Vizzuso, S.; Bosetti, A.; Borsani, B.; Biganzoli, E.; Zuccotti, G. A Multivariate Analysis of “Metabolic Phenotype” Patterns in Children and Adolescents with Obesity for the Early Stratification of Patients at Risk of Metabolic Syndrome. J. Clin. Med. 2022, 11, 1856. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Witczak-Sawczuk, K.; Ostrowska, L.; Cwalina, U.; Leszczyńska, J.; Jastrzębska-Mierzyńska, M.; Hładuński, M.K. Estimation of the Impact of Abdominal Adipose Tissue (Subcutaneous and Visceral) on the Occurrence of Carbohydrate and Lipid Metabolism Disorders in Patients with Obesity-A Pilot Study. Nutrients 2024, 16, 1301. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kurniawan, L.B.; Bahrun, U.; Hatta, M.; Arif, M. Body Mass, Total Body Fat Percentage, and Visceral Fat Level Predict Insulin Resistance Better Than Waist Circumference and Body Mass Index in Healthy Young Male Adults in Indonesia. J. Clin. Med. 2018, 7, 96. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Després, J.P.; Arsenault, B.J.; Côté, M.; Cartier, A.; Lemieux, I. Abdominal obesity: The cholesterol of the 21st century? Can. J. Cardiol. 2008, 24 (Suppl. D), 7D–12D. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Clarke, S.L.; Reaven, G.M.; Leonard, D.; Barlow, C.E.; Haskell, W.L.; Willis, B.L.; DeFina, L.; Knowles, J.W.; Maron, D.J. Cardiorespiratory Fitness, Body Mass Index, and Markers of Insulin Resistance in Apparently Healthy Women and Men. Am. J. Med. 2020, 133, 825–830.e2. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cavaliere, G.; Cimmino, F.; Trinchese, G.; Catapano, A.; Petrella, L.; D’Angelo, M.; Lucchin, L.; Mollica, M.P. From Obesity-Induced Low-Grade Inflammation to Lipotoxicity and Mitochondrial Dysfunction: Altered Multi-Crosstalk between Adipose Tissue and Metabolically Active Organs. Antioxidants 2023, 12, 1172. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ahmed, B.; Sultana, R.; Greene, M.W. Adipose tissue and insulin resistance in obese. Biomed. Pharmacother. 2021, 137, 111315. [Google Scholar] [CrossRef] [PubMed]
- Mentxaka, A.; Gómez-Ambrosi, J.; Ramírez, B.; Rodríguez, A.; Becerril, S.; Neira, G.; Valentí, V.; Moncada, R.; Silva, C.; Unamuno, X.; et al. Netrin-1 Promotes Visceral Adipose Tissue Inflammation in Obesity and Is Associated with Insulin Resistance. Nutrients 2022, 14, 4372. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ghemiș, L.; Goriuc, A.; Minea, B.; Botnariu, G.E.; Mârțu, M.A.; Ențuc, M.; Cioloca, D.; Foia, L.G. Myeloid-Derived Suppressor Cells (MDSCs) and Obesity-Induced Inflammation in Type 2 Diabetes. Diagnostics 2024, 14, 2453. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, Y.; Li, D.; Vermund, S.H. Advantages and Limitations of the Body Mass Index (BMI) to Assess Adult Obesity. Int. J. Environ. Res. Public Health 2024, 21, 757. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ramírez-Manent, J.I.; Jover, A.M.; Martinez, C.S.; Tomás-Gil, P.; Martí-Lliteras, P.; López-González, Á.A. Waist Circumference Is an Essential Factor in Predicting Insulin Resistance and Early Detection of Metabolic Syndrome in Adults. Nutrients 2023, 15, 257. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Radu, F.; Potcovaru, C.G.; Salmen, T.; Filip, P.V.; Pop, C.; Fierbințeanu-Braticievici, C. The Link between NAFLD and Metabolic Syndrome. Diagnostics 2023, 13, 614. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jurczewska, J.; Ostrowska, J.; Chełchowska, M.; Panczyk, M.; Rudnicka, E.; Kucharski, M.; Smolarczyk, R.; Szostak-Węgierek, D. Abdominal Obesity in Women with Polycystic Ovary Syndrome and Its Relationship with Diet, Physical Activity and Insulin Resistance: A Pilot Study. Nutrients 2023, 15, 3652. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Espada, M.C.; Ferreira, C.C.; Gamonales, J.M.; Hernández-Beltrán, V.; Massini, D.A.; Macedo, A.G.; Almeida, T.A.F.; Castro, E.A.; Pessôa Filho, D.M. Body Composition Relationship to Performance, Cardiorespiratory Profile, and Tether Force in Youth Trained Swimmers. Life 2023, 13, 1806. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Muntean, P.; Popa, A.; Miclos-Balica, M.; Schick, F.; Munteanu, O.; Pupazan, V.; Neagu, A.; Neagu, M. Learning Effects in Air Displacement Plethysmography. Life 2023, 13, 1315. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gordito Soler, M.; López-González, Á.A.; Vallejos, D.; Martínez-Almoyna Rifá, E.; Vicente-Herrero, M.T.; Ramírez-Manent, J.I. Usefulness of Body Fat and Visceral Fat Determined by Bioimpedanciometry versus Body Mass Index and Waist Circumference in Predicting Elevated Values of Different Risk Scales for Non-Alcoholic Fatty Liver Disease. Nutrients 2024, 16, 2160. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khan, S.H.; Sobia, F.; Niazi, N.K.; Manzoor, S.M.; Fazal, N.; Ahmad, F. Metabolic clustering of risk factors: Evaluation of Triglyceride-glucose index (TyG index) for evaluation of insulin resistance. Diabetol. Metab. Syndr. 2018, 10, 74. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Duan, M.; Zhao, X.; Li, S.; Miao, G.; Bai, L.; Zhang, Q.; Yang, W.; Zhao, X. Metabolic score for insulin resistance (METS-IR) predicts all-cause and cardiovascular mortality in the general population: Evidence from NHANES 2001–2018. Cardiovasc. Diabetol. 2024, 23, 243. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Widjaja, N.A.; Irawan, R.; Hanindita, M.H.; Ugrasena, I.; Handajani, R. METS-IR vs. HOMA-AD and Metabolic Syndrome in Obese Adolescents. J. Med. Investig. 2023, 70, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Tunç Karaman, S. Insulin resistance in non-diabetic hypothyroid patients: A critical examination of METS-IR as a diagnostic marker. Curr. Med. Res. Opin. 2023, 39, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Mestre-Font, M.; Busquets-Cortés, C.; Ramírez-Manent, J.I.; Tomás-Gil, P.; Paublini, H.; López-González, A.A. Influence of sociodemographic variables and healthy habits on the values of overweight and obesity scales in 386,924 Spanish workers. Acad. J. Health Sci. 2024, 39, 27–35. [Google Scholar] [CrossRef]
- Bazyar, H.; Zare Javid, A.; Masoudi, M.R.; Haidari, F.; Heidari, Z.; Hajializadeh, S.; Aghamohammadi, V.; Vajdi, M. Assessing the predictive value of insulin resistance indices for metabolic syndrome risk in type 2 diabetes mellitus patients. Sci. Rep. 2024, 14, 8917. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pereyra González, I.; Lopez-Arana, S. Usefulness of SPISE Index for Screening and Detection of Early Stages of Insulin Resistance among Chilean Young Adults. Ann. Nutr. Metab. 2023, 79, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Correa-Burrows, P.; Matamoros, M.; de Toro, V.; Zepeda, D.; Arriaza, M.; Burrows, R. A Single-Point Insulin Sensitivity Estimator (SPISE) of 5.4 is a good predictor of both metabolic syndrome and insulin resistance in adolescents with obesity. Front. Endocrinol. 2023, 14, 1078949. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barchetta, I.; Dule, S.; Bertoccini, L.; Cimini, F.A.; Sentinelli, F.; Bailetti, D.; Marini, G.; Barbonetti, A.; Loche, S.; Cossu, E.; et al. The single-point insulin sensitivity estimator (SPISE) index is a strong predictor of abnormal glucose metabolism in overweight/obese children: A long-term follow-up study. J. Endocrinol. Investig. 2022, 45, 43–51. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Manzanero, R.Z.; López-González, A.A.; Tomás-Gil, P.; Paublini, H.; Martínez-Jover, A.; Ramírez-Manent, J.I. Estimation of cardiometabolic risk in 25.030 Spanish kitchen workers. Acad. J. Health Sci. 2023, 38, 101–110. [Google Scholar] [CrossRef]
- Gholami, F.; Karimi, Z.; Samadi, M.; Sovied, N.; Yekaninejad, M.S.; Keshavarz, S.A.; Javdan, G.; Bahrampour, N.; Wong, A.; Clark, C.C.T.; et al. The association between dietary pattern and visceral adiposity index, triglyceride-glucose index, inflammation, and body composition among Iranian overweight and obese women. Sci. Rep. 2023, 13, 13162. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Manzanero, R.Z.; López-González, A.A.; Tomás-Gil, P.; Paublini, H.; Martínez-Jover, A.; Ramírez-Manent, J.I. Cardiometabolic risk assessment in 28300 spanish waiters. Acad. J. Health Sci. 2023, 39, 16–24. [Google Scholar] [CrossRef]
- Stewart, A.; Marfell-Jones, M.; Olds, T.; Ridder, H. International Standards for Anthropometric Assessment; International Society for the Advancement of Kinanthropometry–ISAK: Lower Hutt, New Zealand, 2011. [Google Scholar]
- Fang, H.; Berg, E.; Cheng, X.; Shen, W. How to best assess abdominal obesity. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 360–365. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Allain, C.C.; Poon, L.S.; Chan, C.S.; Richmond, W.; Fu, P.C. Enzymatic determination of total serum cholesterol. Clin. Chem. 1974, 20, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Roeschlau, P.; Bernt, E.; Gruber, W. Enzymatic determination of total cholesterol in serum. Z. Klin. Chem. Klin. Biochem. 1974, 12, 226. [Google Scholar] [PubMed]
- Han, Y.; Zhou, Z.; Zhang, Y.; Zhao, G.; Xu, B. The Association of Surrogates of Insulin Resistance with Hyperuricemia among Middle-Aged and Older Individuals: A Population-Based Nationwide Cohort Study. Nutrients 2023, 15, 3139. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seo, M.W.; Cho, W.; Kim, J.Y. The single point insulin sensitivity estimator (SPISE) index as a predictor of metabolic syndrome in Korean adults. Obes. Res. Clin. Pract. 2023, 17, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Mestre Font, M.; Busquets-Cortés, C.; Ramírez-Manent, J.I.; Tomás-Gil, P.; Paublini, H.; López-González, Á.A. Influence of Sociodemographic Variables and Healthy Habits on the Values of Insulin Resistance Indicators in 386,924 Spanish Workers. Nutrients 2023, 15, 5122. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Águilo Juanola, M.C. Should the financing criteria for medicines for the treatment of smoking in Spain be modified? Acad. J. Health Sci. 2023, 38, 157–162. [Google Scholar] [CrossRef]
- Aguiló Juanola, M.C.; López-González, A.A.; Tomás-Gil, P.; Paublini, H.; Tárraga-López, P.J.; Ramírez-Manent, J.I. Influence of tobacco consumption on the values of different insulin resistance risk scales and non-alcoholic fatty liver disease and hepatic fibrosis scales in 418,343 spanish people. Acad. J. Health Sci. 2024, 39, 9–15. [Google Scholar] [CrossRef]
- Mestre-Font, M.; Busquets-Cortés, C.; Ramírez-Manent, J.I.; Tomás-Gil, P.; Paublini, H.; López-González, A.A. Influence of sociodemographic variables and healthy habits on the values of type 2 diabetes risk scales. Acad. J. Health Sci. 2024, 39, 99–106. [Google Scholar] [CrossRef]
- Lee, H.J.; Lim, Y.H.; Hong, Y.C.; Shin, C.H.; Lee, Y.A. Body Mass Index Changes and Insulin Resistance at Age 4: A Prospective Cohort Study. Front. Endocrinol. 2022, 13, 872591. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tucker, L.A. Insulin Resistance and Biological Aging: The Role of Body Mass, Waist Circumference, and Inflammation. Biomed. Res. Int. 2022, 2022, 2146596. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Song, K.; Lee, E.; Lee, H.S.; Lee, H.; Lee, J.W.; Chae, H.W.; Kwon, Y.J. Comparison of SPISE and METS-IR and Other Markers to Predict Insulin Resistance and Elevated Liver Transaminases in Children and Adolescents. Diabetes Metab. J. 2024, 49, 264–274. [Google Scholar] [CrossRef] [PubMed]
- INEbase/Indicadores de Calidad de Vida. Available online: https://www.ine.es/jaxi/Datos.htm?path=/t00/ICV/dim3/l0/&file=33201.px (accessed on 10 December 2024).
- INEbase/Indicadores de Calidad de Vida. Available online: https://www.ine.es/jaxi/Datos.htm?path=/t00/ICV/dim3/&file=33303.px (accessed on 10 December 2024).
- Stefan, N.; Häring, H.U.; Schulze, M.B. Metabolically healthy obesity: The low-hanging fruit in obesity treatment? Lancet Diabetes Endocrinol. 2018, 6, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Metabolically Healthy Obesity. Endocr. Rev. 2020, 41, bnaa004. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stefan, N. Metabolically Healthy and Unhealthy Normal Weight and Obesity. Endocrinol. Metab. 2020, 35, 487–493. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stefan, N. Causes, consequences, and treatment of metabolically unhealthy fat distribution. Lancet Diabetes Endocrinol. 2020, 8, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Koceva, A.; Herman, R.; Janez, A.; Rakusa, M.; Jensterle, M. Sex- and Gender-Related Differences in Obesity: From Pathophysiological Mechanisms to Clinical Implications. Int. J. Mol. Sci. 2024, 25, 7342. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shao, Y.; Li, L.; Zhong, H.; Wang, X.; Hua, Y.; Zhou, X. Anticipated correlation between lean body mass to visceral fat mass ratio and insulin resistance: NHANES 2011–2018. Front. Endocrinol. 2023, 14, 1232896. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Freeman, A.M.; Acevedo, L.A.; Pennings, N. Insulin Resistance. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 17 August 2023. [Google Scholar] [PubMed]
- Vicente-Herrero, M.T.; Egea Sancho, M.; Ramírez Iñiguez de la Torre, M.V.; López-González, A.A. Visceral Adiposity Index (VAI) and Dysfunctional Adiposity Index (DAI). Relationship with insulin resistance and prediabetes risk. Acad. J. Health Sci. 2024, 39, 25–31. [Google Scholar] [CrossRef]
- Weir, C.B.; Jan, A. BMI Classification Percentile And Cut Off Points. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 26 June 2023. [Google Scholar] [PubMed]
- Salmón-Gómez, L.; Catalán, V.; Frühbeck, G.; Gómez-Ambrosi, J. Relevance of body composition in phenotyping the obesities. Rev. Endocr. Metab. Disord. 2023, 24, 809–823. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chevalier, B.; Lion, G.; Semah, F.; Huglo, D. When and how to evaluate adipose tissue in clinical practice? DEXA, impedancemetry. Ann. Endocrinol. 2024, 85, 173–174. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Kaur Walia, G.; Pal Sachdeva, M.; Gupta, V. Genomics of body fat distribution. J. Genet. 2021, 100, 32. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).