Bone Substitute in Diabetic Foot Osteomyelitis Treatment
Abstract
1. Background
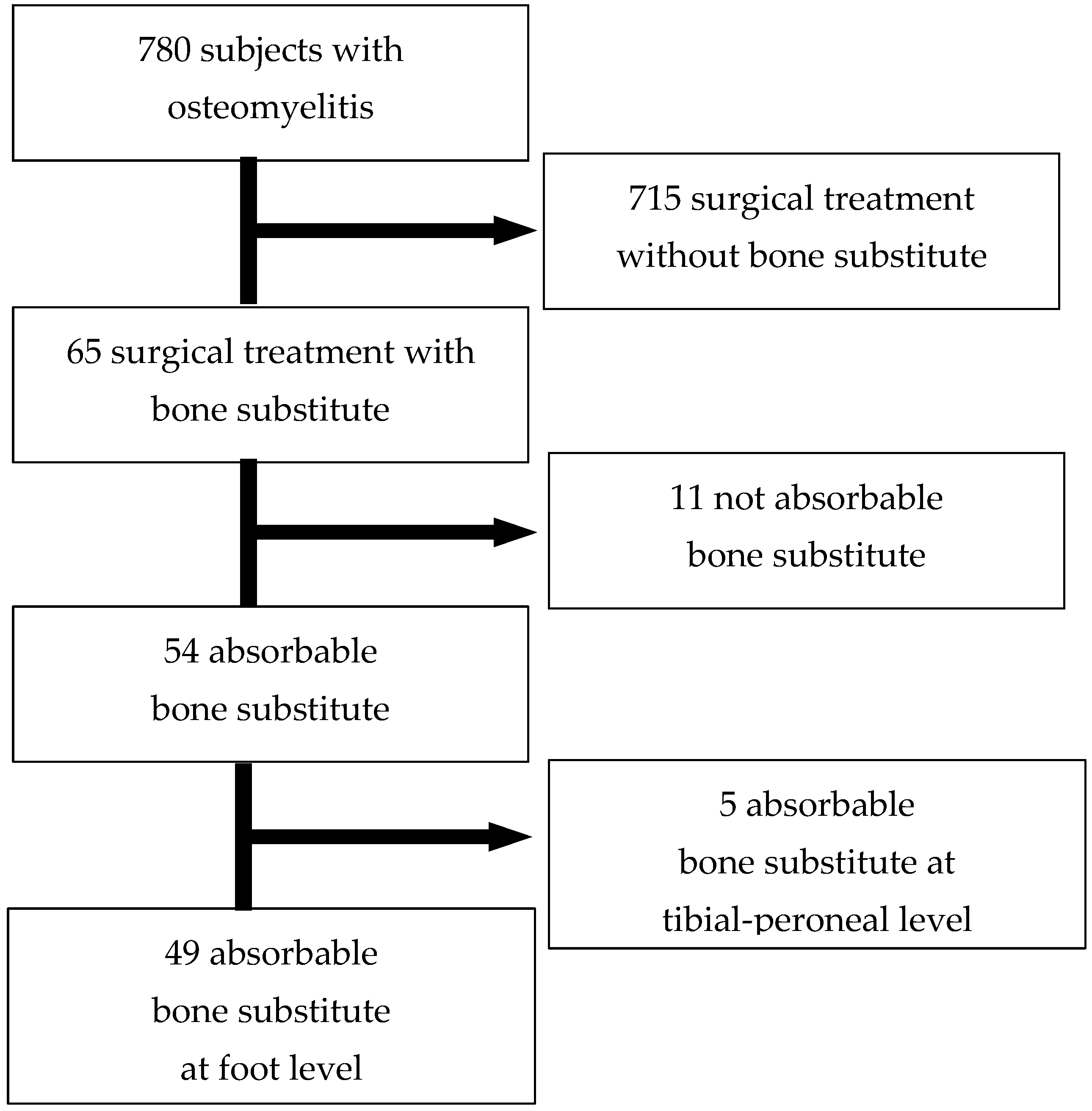
2. Materials and Methods
2.1. Study Design and Participants
2.2. Baseline Data Collection
- -
- sensitivity to a 10 g monofilament: patients were considered to have neuropathy if they did not feel the monofilament at one or more designated test points.
- -
- vibration perception threshold (VPT): This was measured using a biothesiometer (METEDA, San Benedetto del Tronto, Italy). Patients were diagnosed with neuropathy if their VPT was 25 volts or higher at the big toe (hallux) or ankle bone (malleolus) [14].
2.3. Clinical Diagnosis of DFO
2.4. Treatment of DFO
2.4.1. Antibiotic Treatment
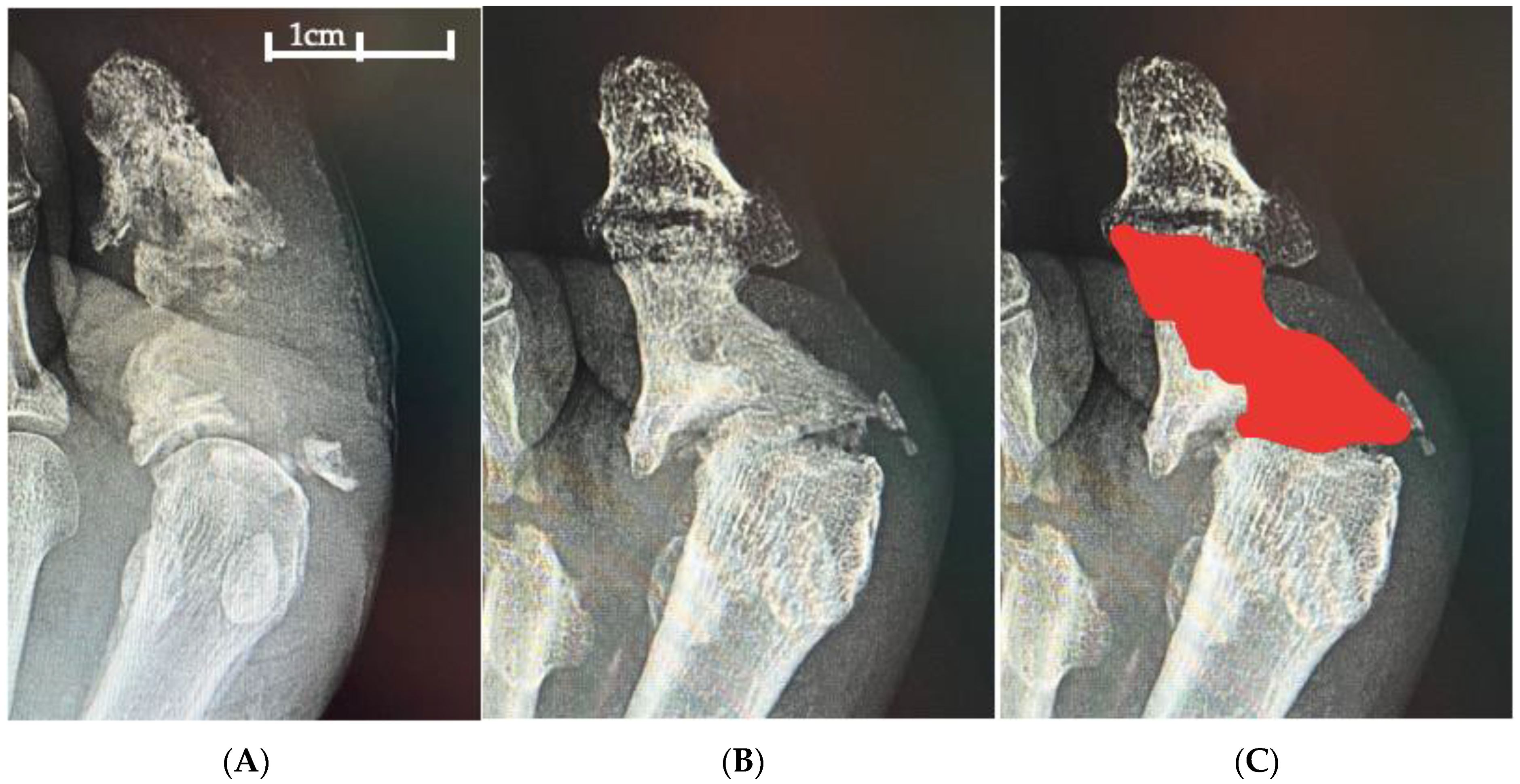
2.4.2. Surgical Intervention
2.4.3. Bone Substitute
2.4.4. Post-Operative Management
2.5. Statistical Analysis
2.6. Ethical Approval
3. Results
3.1. Microbiological Results and Antibiotic Treatment
3.2. Outcome
3.2.1. Lesion Site and Outcome
3.2.2. Bone Substitute Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lipsky, B.A.; Uçkay, I. Treating Diabetic Foot Osteomyelitis: A Practical State-of-the-Art Update. Medicina 2021, 57, 339. [Google Scholar] [CrossRef] [PubMed]
- van Netten, J.J.; Bus, S.A. Definitions and criteria for diabetes-related foot disease (IWGDF 2023 update). Diabetes Metab. Res. Rev. 2023, 15, e3654. [Google Scholar] [CrossRef] [PubMed]
- Lavery, L.A.; Armstrong, D.G. Diabetic foot syndrome: Evaluating the prevalence and incidence of foot pathology in Mexican Americans and non-Hispanic whites from a diabetes disease management cohort. Diabetes Care 2003, 26, 1435–1438. [Google Scholar] [CrossRef] [PubMed]
- Burgess, J.L.; Wyant, W.A. Diabetic Wound-Healing Science. Medicina 2021, 57, 1072. [Google Scholar] [CrossRef]
- Lipsky, B.A.; Berendt, A.R. Infectious Diseases Society of America Clinical Practice Guideline for the Diagnosis and Treatment of Diabetic Foot Infections. Clin. Infect. Dis. 2012, 54, e132–e173. [Google Scholar] [CrossRef]
- Game, F.L.; Jeffcoate, W.J. Primarily non-surgical management of osteomyelitis of the foot in diabetes. Diabetologia 2008, 51, 962–967. [Google Scholar] [CrossRef]
- Da Ros, R.; Assaloni, R. Burden of Infected Diabetic Foot Ulcers on Hospital Admissions and Costs in a Third-Level Center. Diabetology 2024, 5, 141–150. [Google Scholar] [CrossRef]
- Da Ros, R.; Assaloni, R.; Michelli, A.; Brunato, B.; Miranda, C. Antibiotic and Surgical Treatment of Diabetic Foot Osteomyelitis: The Histopathological Evidence. Antibiotics 2024, 13, 1142. [Google Scholar] [CrossRef]
- Lázaro Martínez, J.L.; García Álvarez, Y. Optimal management of diabetic foot osteomyelitis: Challenges and solutions. Diabetes Metab. Syndr. Obes. 2019, 12, 947–959. [Google Scholar] [CrossRef]
- Raghuram, A.; Singh, A.; Chang, D.K.; Nunez, M.; Reece, E.M. Bone Grafts, Bone Substitutes, and Orthobiologics: Applications in Plastic Surgery. Semin. Plast. Surg. 2019, 33, 190–199. [Google Scholar] [CrossRef]
- Schade, V.L.; Roukis, T.S. The role of bone graft and bone graft substitutes in the treatment of infection in the diabetic foot. Podiatry Today 2008, 21, 32–38. [Google Scholar]
- Chatzipapas, C.; Karaglani, M.; Papanas, N.; Tilkeridis, K.; Drosos, G.I. Local Antibiotic Delivery Systems in Diabetic Foot Osteomyelitis: A Brief Review. Rev. Diabet. Stud. 2021, 17, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.; Singh, R.; Agarwal, A.; Wadhwa, R.; Bal, A.; Vaidya, S. Diabetic Foot Ulcers and Osteomyelitis: Use of Biodegradable Calcium Sulfate Beads Impregnated with Antibiotics for Treatment of Multidrug-Resistant Organisms. Wounds 2021, 33, 70–76. [Google Scholar]
- Armstrong, D.G.; Tan, T.W. Diabetic Foot Ulcers: A Review. JAMA 2023, 330, 62–75. [Google Scholar] [CrossRef]
- Hinchliffe, R.J.; Forsythe, R.O.; Apelqvist, J.; Boyko, E.J.; Fitridge, R.; Hong, J.P.; Katsanos, K.; Mills, J.L.; Nikol, S.; Reekers, J.; et al. Guidelines on diagnosis, prognosis, and management of peripheral artery disease in patients with foot ulcers and diabetes (IWGDF 2019 update). Diabetes/Metab. Res. Rev. 2020, 36, e3276. [Google Scholar] [CrossRef]
- Monteiro-Soares, M.; Hamilton, E.J.; Russell, D.A.; Srisawasdi, G.; Boyko, E.J.; Mills, J.L.; Jeffcoate, W.; Game, F. Guidelines on the classification of foot ulcers in people with diabetes (IWGDF 2023 update). Diabetes/Metab. Res. Rev. 2024, 40, e3648. [Google Scholar] [CrossRef]
- Schmidt, B.M.; Jarocki, C. Making the equivocal unequivocal: Standardization of clean margins in diabetic foot osteomyelitis. Clin. Diabetes Endocrinol. 2020, 6, 8. [Google Scholar] [CrossRef]
- Hench, L.L. The story of Bioglass. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef]
- Winkler, E.; Schöni, M.; Krähenbühl, N.; Uçkay, I.; Waibel, F.W.A. Foot Osteomyelitis Location and Rates of Primary or Secondary Major Amputations in Patients with Diabetes. Foot Ankle Int. 2022, 43, 957–967. [Google Scholar] [CrossRef]
- Whisstock, C.; Volpe, A.; Ninkovic, S.; Marin, M.; Meloni, M.; Bruseghin, M.; Boschetti, G.; Brocco, E. Multidisciplinary Approach for the Management and Treatment of Diabetic Foot Infections with a Resorbable, Gentamicin-Loaded Bone Graft Substitute. J. Clin. Med. 2020, 9, 3586. [Google Scholar] [CrossRef]
- Niazi, N.S.; Drampalos, E.; Morrissey, N.; Jahangir, N.; Wee, A.; Pillai, A. Adjuvant antibiotic loaded bio composite in the management of diabetic foot osteomyelitis—A multicentre study. Foot 2019, 39, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Hutting, K.H.; Aan de Stegge, W.B. Surgical Treatment of Diabetic Foot Ulcers Complicated by Osteomyelitis with Gentamicin-Loaded Calcium Sulphate-Hydroxyapatite Biocomposite. J. Clin. Med. 2021, 19, 371. [Google Scholar] [CrossRef] [PubMed]
- Monami, M.; Bordoni, L. Efficacy and safety of a bio-absorbable antibiotic delivery in calcium sulphate granules for the treatment of osteomyelitis in patients with diabetic foot: A randomized, double blinded, controlled clinical study The BIG D-FOOT study. Diabetes Obes. Metab. 2025, 27, 2552–2560. [Google Scholar] [CrossRef] [PubMed]
- De Giglio, R.; Di Vieste, G. Efficacy and Safety of Bioactive Glass S53P4 as a Treatment for Diabetic Foot Osteomyelitis. J. Foot Ankle Surg. 2021, 60, 292–296. [Google Scholar] [CrossRef]
- Pickwell, K.M.; Siersma, V.D.; Kars, M.; Holstein, P.E.; Schaper, N.C.; on behalf of the Eurodiale consortium. Diabetic foot disease: Impact of ulcer location on ulcer healing. Diabetes/Metab. Res. Rev. 2013, 29, 377–383. [Google Scholar] [CrossRef]
- Khoo, R.; Jansen, S. Slow to heel: A literature review on the management of diabetic calcaneal ulceration. Int. Wound J. 2018, 15, 205–211. [Google Scholar] [CrossRef]
- Meloni, M.; Giurato, L.; Monge, L.; Miranda, C.; Scatena, A.; Ragghianti, B.; Silverii, G.A.; Vermigli, C.; De Cassai, A.; Volpe, A.; et al. Effect of a multidisciplinary team approach in patients with diabetic foot ulcers on major adverse limb events (MALEs): Systematic review and meta-analysis for the development of the Italian guidelines for the treatment of diabetic foot syndrome. Acta Diabetol. 2024, 61, 543–553. [Google Scholar] [CrossRef]
- Thabit, A.K.; Fatani, D.F. Bone graft in diabetic foot osteomyelitis: A review. Int. Surg. J. 2017, 4, 2601–2606. [Google Scholar]
- Schade, V.L.; Roukis, T.S. The role of polymethylmethacrylate antibiotic–loaded cement in addition to debridement for the treatment of soft tissue and osseous infections of the foot and ankle. J. Foot Ankle Surg. 2010, 49, 55–62. [Google Scholar] [CrossRef]
- Uddin, A.; Russell, D.; Game, F.; Santos, D.; Siddle, H.J. The effectiveness of systemic antibiotics for osteomyelitis of the foot in adults with diabetes mellitus: A systematic review protocol. J. Foot Ankle Res. 2022, 15, 48. [Google Scholar] [CrossRef]
- Soldevila-Boixader, L.; Fernández, A.P.; Laguna, J.M.; Uçkay, I. Local Antibiotics in the Treatment of Diabetic Foot Infections: A Narrative Review. Antibiotics 2023, 12, 124. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Hatch, D.; Wells, C.; Ahn, D.; Harris, M.; Jennings, J.; Haggard, W.; Armstrong, D. Characteristics and clinical assessment of antibiotic delivery by chitosan sponge in the high-risk diabetic foot: A case series. J. Wound Care 2017, 26, S32–S38. [Google Scholar] [CrossRef] [PubMed]
- Lavery, L.A.; Tarricone, A.N. Does complete resection of infected bone improve clinical outcomes in patients with diabetic foot osteomyelitis? Int. Wound J. 2024, 21, e70072. [Google Scholar] [CrossRef] [PubMed]
- McNally, M.A.; Ferguson, J.Y.; Lau, A.C.; Diefenbeck, M.; Scarborough, M.; Ramsden, A.J.; Atkins, B.L. Single-stage treatment of chronic osteomyelitis with a new absorbable, gentamicin-loaded, calcium sulphate/hydroxyapatite biocomposite: A prospective series of 100 cases. Bone Jt. J. 2016, 98, 1289–1296. [Google Scholar] [CrossRef]
- Miranda, C.; Da Ros, R. Prevention of Diabetic Foot Ulcer: A Neglected Opportunity. Transl. Med. UniSa 2020, 22, 50–51. [Google Scholar]
- Da Ros, R.; Volpe, A.; Bordieri, C.; Tramonta, R.; Bernetti, A.; Scatena, A.; Monge, L.; Ragghianti, B.; Silverii, A.; Uccioli, L.; et al. Prevention of foot ulcers recurrence in patients with diabetes: A systematic review and meta-analysis of randomized controlled trials for the development of the italian guidelines for the treatment of diabetic foot syndrome. Acta Diabetol. 2024, 61, 1363–1373. [Google Scholar] [CrossRef]
- Uivaraseanu, B.; Bungau, S. Clinical, Pathological and Microbiological Evaluation of Diabetic Foot Syndrome. Medicina 2020, 56, 380. [Google Scholar] [CrossRef]
| Commercial Product | Composition | Absorbable | Osteoinduction | Antibiotic (n. Treated) | Subject Treated |
|---|---|---|---|---|---|
| Cerament® G Bonesupport, Lund, Schweden | Calcium sulphate (60%) Hydroxyapatite (40%) | Yes | Yes | GEN | 4 (7%) |
| Stimulan® Biocomposite Ltd. Staffordshire, UK | Calcium sulphate (100%) | Yes | Yes | GEN | 1 (2%) |
| BioSphere® Putty Synergy Biomedical, Wayne, PA, USA | Bimodal bioactive glass spheres in a resorbable phospholipid carrier | Yes | Yes | No | 19 (39%) |
| PerOssal® OSARTIS, Münster, Germany | 51.5% nanocrystalline hydroxyapatite 48.5% calcium sulfate | Yes | Yes | GEN (4) GEN-VAN (18) | 24 (50%) |
| Bone Chips Bioteck, Arcugnano, Italy | Bone chips 100% | No | Yes | GEN-VAN | 1 (2%) |
| Characteristics | All 49 Subjects Mean ± SD | Forefoot/Midfoot 27 Subjects (55%) Mean ± SD | Rearfoot 22 Subjects (45%) Mean ± SD | P (Forefoot/Midfoot vs. Rearfoot) |
|---|---|---|---|---|
| Age (years) | 66 ± 10 years | 66 ± 12 | 66 ± 9 | 0.89 |
| DM history (years) | 18 ± 13 | 17 ± 13 | 21 ± 13 | 0.14 |
| Male/female n. | 43/6 | 24/3 | 19/3 | 0.79 |
| HbA1c (%) | 7.9 ± 1.7 | 7.7 ± 1.9 | 8.1 ± 1.7 | 0.45 |
| Peripheral neuropathy n (%) | 46 (94%) | 25 (93%) | 21 (95%) | 0.68 |
| Peripheral vascular disease n (%) | 24 (49%) | 13 (48%) | 11 (50%) | 0.9 |
| Revascularization | 24 (49) | 13 (48%) | 11 (50%) | 0.9 |
| Dyalisis | 2 (4%) | 2 (7%) | 0 | 0.2 |
| Lesion Texas 3A n (%) | 17 (34%) | 10 (37%) | 7 (32%) | 0.7 |
| Lesion Texas 3B n (%) | 8 (16%) | 4 (15%) | 4 (18%) | 0.8 |
| Lesion Texas 3C n (%) | 14 (28%) | 9 (33%) | 5 (23%) | 0.4 |
| Lesion Texas 3D n (%) | 10 (20%) | 4 (15%) | 6 (27%) | 0.3 |
| Healed n. | 34 | 23 | 11 | <0.01 |
| Healing rate | 69% | 85% | 50% | <0.01 |
| Healing time (months) | 2.3 (Q1 1.5, Q3 5) | 2.7 (Q1 1.7, Q3 5) | 2.2 (Q1 1.2, Q3 3.7) | 0.49 |
| Infective relapse n (%) | 11 (22%) | 4 (15%) | 7 (32%) | 0.2 |
| Characteristics | No Antibiotic 21 Subjects (43%) Mean ± SD | Local Antibiotic 28 Subjects (57%) Mean ± SD | P |
|---|---|---|---|
| Age (years) | 69 ± 13 | 65 ± 10 | 0.16 |
| DM history (years) | 19 ± 13 | 16 ± 12 | 0.2 |
| Male/female n. | 18/3 | 25/3 | 0.71 |
| HbA1c (%) | 8.2 ± 1.9 | 7.8 ± 1.7 | 0.43 |
| Peripheral neuropathy n (%) | 21 (100) | 25 (91%) | 0.12 |
| Peripheral vascular disease n (%) | 15 (71%) | 9 (32%) | 0.005 |
| Revascularization n (%) | 15 (71%) | 9 (32%) | 0.005 |
| Dyalisis n (%) | 2 (9%) | 0 | 0.1 |
| Healed n (%) | 13 (62%) | 21 (75%) | 0.33 |
| Healing time (months) | 2.7 (Q1 1.5, Q3 6) | 2.3 (Q1 1.7, Q3 4) | 0.14 |
| Infective relapse n (%) | 7 (33) | 4 (14) | 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Da Ros, R.; Assaloni, R.; Michelli, A.; Brunato, B.; Barro, E.; Miranda, C. Bone Substitute in Diabetic Foot Osteomyelitis Treatment. Diabetology 2025, 6, 30. https://doi.org/10.3390/diabetology6040030
Da Ros R, Assaloni R, Michelli A, Brunato B, Barro E, Miranda C. Bone Substitute in Diabetic Foot Osteomyelitis Treatment. Diabetology. 2025; 6(4):30. https://doi.org/10.3390/diabetology6040030
Chicago/Turabian StyleDa Ros, Roberto, Roberta Assaloni, Andrea Michelli, Barbara Brunato, Enrica Barro, and Cesare Miranda. 2025. "Bone Substitute in Diabetic Foot Osteomyelitis Treatment" Diabetology 6, no. 4: 30. https://doi.org/10.3390/diabetology6040030
APA StyleDa Ros, R., Assaloni, R., Michelli, A., Brunato, B., Barro, E., & Miranda, C. (2025). Bone Substitute in Diabetic Foot Osteomyelitis Treatment. Diabetology, 6(4), 30. https://doi.org/10.3390/diabetology6040030








